
Acute exacerbation of interstitial lung diseases secondary to systemic rheumatic diseases: a prospective study and review of the literature
Introduction
Interstitial lung disease (ILD) represents one of the most frequent complication of many systemic autoimmune rheumatic diseases, mainly rheumatoid arthritis (RA), connective tissue diseases (CTDs), and more rarely vasculitides. The clinical course of ILD secondary to CTD or vasculitis is variable, ranging from a slow progressive decline of lung function over time to a rapidly progressive course (1).
Some patients with ILD experience an acute idiopathic respiratory deterioration called acute exacerbation (AE) associated to very high mortality (2) and described for the first time in patients with idiopathic pulmonary fibrosis (IPF) (3). AE has also been reported in various forms of ILD other than IPF, included ILD secondary to systemic autoimmune rheumatic diseases (4-9).
However, the number of reported cases of AE in CTD-ILD and vasculitis-ILD is very small, and available literature data doesn’t clarify the real clinical impact of this severe complication in CTD patients (10).
Aim of our study was to prospectively investigate incidence, clinical features and outcome of AE in a population of patients with ILD secondary to systemic inflammatory rheumatic diseases, namely CTD, RA, and vasculitis.
Patients and methods
During a 24 month-period (January 1st, 2014–December 31st, 2015), we enrolled in a cross-sectional study all consecutive patients affected by ILD secondary to systemic autoimmune rheumatic diseases, both incident and prevalent cases, referring to our clinic dedicated to rare lung diseases (11-14). All patients were evaluated by a multidisciplinary team, composed by rheumatologist, pulmonologist and radiologist, with a long-term experience on clinical aspects and treatment of both ILDs and rheumatic diseases. The study was approved by the local ethical committee and all patients signed a consent form before to enter in the study.
Assessment of rheumatic disease
At baseline, all patients underwent to a core set of serum autoantibodies and laboratory investigations; data about demographic, disease onset, clinical, serological and therapeutic features were also recorded. The initial patient’s assessment was followed by a periodical re-evaluation until December 31, 2016. If AE occurred, its clinical course and outcome were also reported.
Rheumatic diseases were classified according to the current international classification criteria: in particular, Systemic sclerosis (SSc) according to 2013 Classification Criteria (11), Sjogren syndrome (SS) according to 2002 or 2016 ACR/EULAR Sjögren’s Syndrome Classification Criteria (12), inflammatory muscle diseases were classified according to Bohan and Peter criteria (13), and RA was classified according to 2010 ACR/EULAR RA Classification Criteria (14). For the ANCA associated vasculitides we used the 1990 Criteria for Churg-Strauss Syndrome (formerly eosinophilia with Granulomatosis with Polyangiitis) and Wegener granulomatosis (formerly Granulomatosis with Polyangiitis) (15,16). Finally, the preliminary research criteria for the “interstitial pneumonia with autoimmune features” (IPAF) were used to classify patients who have ILD with autoimmune features, but not fulfill the specific criteria for CTD (17).
Assessment of lung involvement
Diagnosis of ILD was made by mean of high-resolution computed tomography (HRCT). All images were scored by a thoracic radiologist experienced in ILD, classifying them as definite, possible or inconsistent with usual interstitial pneumonia (UIP) pattern (1). If an inconsistent with UIP pattern was noted, the pattern was furtherly classified in nonspecific interstitial pneumonia (NSIP), organizing pneumonia (OP), mixed pattern NSIP/OP or lymphoid interstitial pneumonia (18), classifying patients according to the prevalent pattern. The result of pulmonary function tests was expressed as the percentage of the predicted value of each parameter and corrected for age, gender and height. Single-breath diffusing capacity of the lung for carbon monoxide (DLCO) was used to assess gas transfer. Clinical evaluation and pulmonary function tests were repeated every 3 months or when a deterioration of clinical status occurred.
Diagnosis of AE
In absence of dedicated diagnostic criteria for AE in ILD related to rheumatic disorders, we adopted the revised diagnostic criteria for AE-IPF (3): acute worsening or development of dyspnea typically of less than 1-month duration; HRCT with new bilateral ground-glass opacities and/or consolidations superimposed on a background pattern consistent with UIP pattern; deterioration not fully explained by cardiac failure or fluid overload. As previously suggested, the occurrence of this clinical and radiological picture in a background of possible or inconsistent with UIP pattern was also considered diagnostic for AE (10). When clinically suggestive, blood β-D glucan and cytomegalovirus antigen were also searched, while quantiferon test was performed in all patients.
When possible, for all patients, bronchoscopy with washings or bronchoalveolar lavage (BAL) was performed to exclude infections. C-reactive protein, blood cultures and sputum cultures were also performed in all patients.
Statistical analysis
Categorical variables were analyzed by chi square test or Fisher’s exact test and differences between the means were determined using Mann-Whitney test for unpaired samples. Clinical features were considered as dichotomic or ordinal parameters (HRCT patterns, sex, antinuclear antibodies, smoke); for DLCO and forced vital capacity (FVC) a cut-off of 47% and 75% was identified according to previous data (16). Finally, age, durations of rheumatic disease and ILD were considered as continuous values. P values<0.05 were considered statistically significant. To define the possible predictive factors of AE only baseline values were considered. Cox regression was performed to analyze the effect of the baseline features of the patients on development of AE and survival and cumulative survival rates were computed by the Kaplan-Meier method (19).
Results
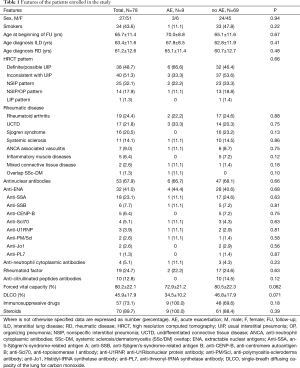
Seventy-eight patients with ILD associated to systemic autoimmune disease (mean age 65.7±11.4, female/male 51/27) were enrolled in the study, and observed for a mean follow-up period of 23.9±10.9 months (range, 12–36 months) (Table 1).
Full table
Diagnosis of ILD was contemporary to the rheumatic disease in 18/78 (23.1%) patients; ILD preceded the diagnosis of the rheumatic disease in 29/78 (37.2%) subjects, while in the remaining 31 (39.7%) patients ILD occurred during the course of the rheumatic disease. At baseline, the mean duration of rheumatic disease and ILD was 52.5±75.4 and 25.8±35.3 months, respectively.
A definite or possible UIP pattern was observed in 48.7% of cases, while an inconsistent with UIP pattern was recorded in 51.3%. Antinuclear antibodies (ANA) were positive in 67.9% of subjects and extractable nuclear antigens in 41.1%, mainly anti-SSA antibodies (Table 1).
Finally, 57 patients (73.1%) underwent immunosuppressive drugs, while steroids were administered in 70 patients (89.8%), prevalently at low dosage (prednisolone ≤5 mg daily).
Patients with AE
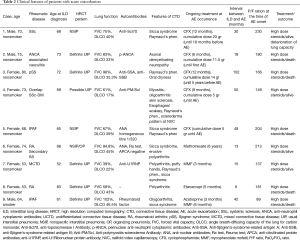
Nine patients experienced an AE, with an estimated incidence of 5.77 AE/100 patients/year. In a patient, a relapse of AE was observed 4 months after the first episode. On average, AE appeared 73±76.6 months after diagnosis of CTD or vasculitis and 41.7±28.9 months after diagnosis of ILD. Clinical and serological features of patients with AE are described in Table 2. Triggering factors such as infections were excluded in all patients by mean of serology and BAL and no patients had a recent history of infection. Cytological analysis of BAL fluid revealed non-specific presence of low levels of neutrophils and lymphocytes, thus reinforcing the exclusion of infection. When performed blood β-D glucan and cytomegalovirus antigen were negative, as well as quantiferon test. Moreover, none of the patients that experienced AE showed a BAL fluid compatible with alveolar hemorrhage. None of the patient experienced further deterioration of respiratory failure after BAL had been performed. Only in one patient (patient 2, Table 2) the AE could be triggered by a lung biopsy. At baseline, DLCO was lower in patients developing AE (46.8±17.9 vs. 34.5±10.2, respectively; P not significant); however, a DLCO <47% was recorded in all patients with AE versus 52.2% of the patients without AE (P=0.018). A definite or possible UIP pattern was observed in 6/9 AE cases, while 2 patients showed a NSIP pattern and one a mixed NSIP/OP pattern (not significant); No other significant differences were observed between patients with or without AE, including the frequency of previous use of immunosuppressive drugs or steroids and the type of rheumatic disease (Table 1). The baseline value of DLCO was the only factor associated to the development of AE at univariate analysis, also confirmed at multivariate Cox regression, with 1.057-fold reduction of the risk for every 1%-increase of DLCO (HR: 0.946; 95% CI, 0.897–0.997; P=0.037).
Full table
Outcome
All patients with AE were hospitalized in a Respiratory Intensive Care Unit and treated with high dose steroids (6-metyl-prednisone 500 mg daily for 3 days, followed by 1 mg/kg daily), O2 therapy and life-support treatment (3,8).
Four patients died within 1 month by AE; one patient died 4 months after the first episode of AE because of a relapse, despite anti-CD20 therapy. Two of three survived patients recovered their previous lung function, while the third patient showed a significant reduction of lung function after AE.
Overall, 12 patients died during the follow-up, and AE was the main cause of death in 5 patients; 3 other subjects died for infections, 2 for the evolution of the lung disease, the last 2 for the complications of a lung transplantation and for aspiration pneumonia. Survival of patients with and without AE was summarized in Figure 1. The baseline value of DLCO was the only predictive factor of death with a reduction of the risk of 0.946 for every 1%-increase of DLCO (HR: 0.951; 95% CI, 0.911–0.992; P=0.02).
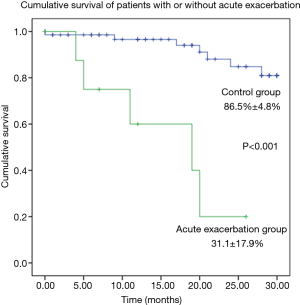
A baseline valued of DLCO <47% was associated to a 9.77-fold increase of the risk of death (95% CI, 1.57–60.97).
Finally, the patient 9 died in January 2017, one month after an AE occurred in December 2016 (Table 2).
Discussion
Our study aimed to investigate prospectively the incidence, clinical features and outcome of AE in a population of ILD related to CTD (including RA) and vasculitis.
AE is a severe complication of interstitial pneumonia, mainly described in IPF patients with a heterogeneous incidence rate (3,4,20-22). A recent meta-analysis of 6 clinical trials revealed a weighted average of 41 AE per 1,000 patients/year in IPF patients, showing a higher incidence in cohort studies than in clinical trials, from 86 to 142 per 1,000 patients-years (23). AE deeply influences the prognosis of the patients with IPF and up to 46% of deaths in IPF are preceded by an AE (23).
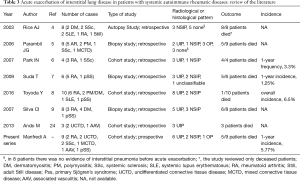
More recently, the occurrence of AE was recorded in other forms of interstitial pneumonia than IPF, but nowadays, less than 50 cases of AE of ILD associated to autoimmune systemic rheumatic diseases have been retrospectively described (Table 3; 4-9,23).
Full table
In 2003, Rice et al. described for the first time 8 cases of AE in CTD (4). Subsequently, a case series of 9 consecutive patients with CTD and histologically proved diffuse alveolar damage was described, reinforcing the hypothesis that AE can complicate the clinical course of patients with CTD-related ILD (5).
In 2007, Park et al. reported 4/93 AE occurred in patients with CTD-ILD with a frequency of 3.3% per year; in two cases the AE occurred after surgical biopsy and all patients died (6). Similarly, in 2009 Suda reported the death of 5/6 patients with AE in a cohort of 83 CTD-ILD (7).
Toyoda et al. retrospectively described 56 cases of acute deterioration of respiratory conditions in 155 CTD-ILD patients, and 10 of them were diagnosed as affected by AE with a prevalence of 6.5%; only one patient died (8).
Recently, several retrospective case series have described the association of ILD and anti-neutrophil cytoplasm antibodies (ANCA) associated vasculitis (AAV), particularly anti-myeloperoxidase ANCA (24). In the majority of these patients, pulmonary fibrosis occurs concurrently or precedes the diagnosis of AAV (25) The clinical history of ILD in these patients didn’t differ by that described in other forms of secondary ILD. At our knowledge, our patient number 2 is the second case of AE described in ILD secondary to AAV (26).
Our study showed an estimated incidence of AE of 5.77 AE/100 patients/year and this data was in line with that reported for IPF patients (3,21-23), but larger studies need to confirm these data. In our population, AE was more frequently observed in patients with UIP pattern, but also detectable in patients with different radiologic patterns (p not significant).
In the present study, AE has proved to be a life-threatening complication and, as previously described in IPF, it represents the first cause of death, followed by infective complications (3,25).
Of course, this paper presents some limits, mainly the short period of observation, the low number of patients, and the heterogeneity of the enrolled group.
Diagnosis of AE of ILD in the context of rheumatic disease is quite difficult in clinical practice. Many confusing factors have to be considered, such as opportunistic infections. To avoid misdiagnosis, all patients with AE in our study underwent BAL to exclude infections.
Further large studies could better clarify possible predictive risks of AE in patients with CTDs, RA, and vasculitides, and evaluate possible differences among the single rheumatic diseases. Our data suggest to carefully considering this life-threatening complication as a possible natural course of ILD secondary to rheumatic inflammatory disease, such as for IPF patients. This could allow an early diagnosis and a prompt management, in particular in patients with lower DLCO.
Acknowledgements
A grant of 60.000 Euro from FAR (Fondo di Ateneo per la Ricerca) by University of Modena and Reggio Emilia has allowed the study.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Tachikawa R, Tomii K, Ueda H, et al. Clinical features and outcome of acute exacerbation of interstitial pneumonia: collagen vascular diseases-related versus idiopathic. Respiration 2012;83:20-7. [Crossref] [PubMed]
- Collard HR, Ryerson CJ, Corte TJ, et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am J Respir Crit Care Med 2016;194:265-75. [Crossref] [PubMed]
- Rice AJ, Wells AU, Bouros D, et al. Terminal diffuse alveolar damage in relation to interstitial pneumonias. An autopsy study. Am J Clin Pathol 2003;119:709-14. [Crossref] [PubMed]
- Parambil JG, Myers JL, Ryu JH. Diffuse alveolar damage: uncommon manifestation of pulmonary involvement in patients with connective tissue diseases. Chest 2006;130:553-8. [Crossref] [PubMed]
- Park IN, Kim DS, Shim TS, et al. Acute exacerbation of interstitial pneumonia other than idiopathic pulmonary fibrosis. Chest 2007;132:214-20. [Crossref] [PubMed]
- Suda T, Kaida Y, Nakamura Y, et al. Acute exacerbation of interstitial pneumonia associated with collagen vascular diseases. Respir Med 2009;103:846-53. [Crossref] [PubMed]
- Toyoda Y, Hanibuchi M, Kishi J, et al. Clinical features and outcome of acute exacerbation of interstitial pneumonia associated with connective tissue disease. J Med Invest 2016;63:294-9. [Crossref] [PubMed]
- Silva CI, Müller NL, Fujimoto K, et al. Acute exacerbation of chronic interstitial pneumonia: high-resolution computed tomography and pathologic findings. J Thorac Imaging 2007;22:221-9. [Crossref] [PubMed]
- Papanikolaou IC, Drakopanagiotakis F, Polychronopoulos VS. Acute exacerbations of interstitial lung diseases. Curr Opin Pulm Med 2010;16:480-6. [Crossref] [PubMed]
- van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum 2013;65:2737-47. [Crossref] [PubMed]
- Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American–European consensus group. Ann Rheum Dis 2002;61:554-8. [Crossref] [PubMed]
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975;292:344-7. [Crossref] [PubMed]
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American college of rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569-81. [Crossref] [PubMed]
- Masi AT, Hunder GG, Lie JT, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum 1990;33:1094-100. [Crossref] [PubMed]
- Leavitt RY, Fauci AS, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Wegener's granulomatosis. Arthritis Rheum 1990;33:1101-7. [Crossref] [PubMed]
- Fischer A, Antoniou KM, Brown KK, et al. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J 2015;46:976-87. [Crossref] [PubMed]
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Altman DG. Practical statistic for Medical Research. London: Chapman & Hall al; 1991.
- Mura M, Porretta MA, Bargagli E, et al. Predicting survival in newly diagnosed idiopathic pulmonary fibrosis: a 3-year prospective study. Eur Respir J 2012;40:101-9. [Crossref] [PubMed]
- Luppi F, Cerri S, Taddei S, et al. Acute exacerbation of idiopathic pulmonary fibrosis: a clinical review. Intern Emerg Med 2015;10:401-11. [Crossref] [PubMed]
- Atkins CP, Loke YK, Wilson AM. Outcomes in idiopathic pulmonary fibrosis: a meta-analysis from placebo controlled trials. Respir Med 2014;108:376-87. [Crossref] [PubMed]
- Kondoh Y, Taniguchi H, Kawabata Y, et al. Acute exacerbation in idiopathic pulmonary fibrosis. Analysis of clinical and pathologic findings in three cases. Chest 1993;103:1808-12. [Crossref] [PubMed]
- Ando M, Miyazaki E, Ishii T, et al. Incidence of myeloperoxidase anti-neutrophil cytoplasmic antibody positivity and microscopic polyangitis in the course of idiopathic pulmonary fibrosis. Respir Med 2013;107:608-15. [Crossref] [PubMed]
- Kondoh Y, Taniguchi H, Katsuta T, et al. Risk factors of acute exacerbation of idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis 2010;27:103-10. [PubMed]
- Alba MA, Flores-Suárez LF, Henderson AG, et al. Interstital lung disease in ANCA vasculitis. Autoimmun Rev 2017;16:722-9. [Crossref] [PubMed]


