
Endoscopic and surgical treatment for bronchopleural fistula after major lung resection: an enduring challenge
Bronchopleural fistula (BPF) is a serious and potentially fatal complication of lung resection surgery (1). It’s incidence ranges from 4.5% to 20% after pneumonectomy and is estimated at 0.5% after lobectomy; such complication has a remarkable impact on mortality in post-op setting with a mortality rate of 27% in the more recent series and up to 70% in the previous ones (2-4).
The pathogenesis is mainly due to a defect in the bronchial stump healing this resulting in partial up to complete ischemic necrosis of bronchial tissue and seldom in a stump invasion by residual neoplastic tissue (5). The pathological connection between the airway and the pleural space often brings to the onset of empyema and pneumonia sometimes associated with respiratory distress.
BPF occurs with higher incidence in presence of risk factors such as predisponent clinical conditions (diabetes, steroids), previous administration of chemo and/or radiotherapy, defects of surgical technique (excessive skeletonization of bronchial tree and length of bronchial stump and neoplastic residue on the stump (6-8). There is a certain body of evidence (6,7) suggesting that extended lymphadenectomy could result in a devascularization of the bronchial stump and, as a consequence of this, in an impaired bronchial stump healing.
Several coverage techniques have been assessed for the protection of the bronchial stump. These seem to give a protection against the onset of the bronchial fistula and may include various kind of tissue such as pedicled muscular, pericardial, fat and pleural flaps (9). Nevertheless, bronchial stump dehiscence remains one of most feared and often unavoidable complication following major lung resections.
Size of defect and timing of BPF onset are two critical points for its prognosis and management. Large bronchial defects are often due to massive ischemic bronchial necrosis and could represent a life-threatening condition especially after pneumonectomy (10). They often require an extensive surgical intervention because of the difficulties of re-suturing, due to the tissue frailty. Smaller bronchial lesions are better tolerated and a conservative and/or endoscopic treatment could be considered in these cases as an available option as an alternative to surgical reexploration.
Classically, the BPF is classified (11) according to the time of its onset in: (I) ‘‘early’’ if it occurs from 1 to 7 days after surgery; (II) ‘‘intermediate’’ (from 8 to 30 days after surgery) and (III) ‘‘late’’ (more than 30 days after surgery). Early BPF is most commonly due to surgical technical problems (stump insufficiency because of stitches or staples malfunctioning) or acute ischemia of bronchial stump because of devascularization for proximal dissection and/or extended lymphadenectomy; the clinical scenario generally consists of respiratory distress, pneumothorax (in case of lobectomy), air-enlarged post-pneumonectomy space, and subcutaneous and/or mediastinal emphysema. Fever and septic complications may be present when fistula becomes evident or may appear later when infection of pleural space occurs. If contralateral pneumonia occurs, respiratory failure is the rule and mechanical ventilation may be required. In case of early BPF, urgent pleural drainage and supportive measures in an ICU setting (including, if indicated invasive mechanical ventilation with selective bronchial intubation) are the urgent life-saving measures. Once clinical stabilization achieved, a surgical revision and attempt of re-suturing the bronchial stump and protection by muscular flap transposition would theoretically offer the better chance of success, especially in “very early” (within the 3rd postoperative day) BPF which are more likely due to a technical problem. A part these rare presentations, for the remaining early BPF three surgical strategies are possible, when conservative management fails, namely iterative thoracotomy and re-suturing, or open drainage followed by thoracoplasty, or up-front thoracoplasty (generally Andrews) if patient is sufficiently fit and respiratory conditions and sepsis fully stabilized.
The presentation of intermediate and late BPFs are different, usually consisting of infection of the pleural cavity (aspiration pneumonia and empyema), weight loss, and poor general health. The cause is often the chronic ischemia suffering of bronchial tissue or neoplastic erosion of bronchial stump. Patient related factors (i.e., advanced age, malnutrition, ongoing pulmonary or pleural infection, and recurrence of malignancy) may play a crucial role in these cases, probably even more than in early BPF. It’s a common observation that the bronchial necrosis involves also the pars membranacea and surgical approaches are often unsatisfactory in these cases (8).
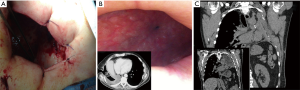
Surgical approaches may range from re-suturing and covering of the bronchial stump by muscle flap apposition up to a more “aggressive” completion pneumonectomy (in case of post-lobectomy fistula). Transternal access and bronchial re-suturing (12) seems to ensure a better closure of the bronchial stump far from the infected tissue, especially in case of BPF complicating a left pneumonectomy, in which the access at bronchial stump is hard through transthoracic access, but this is indicated only if the bronchial stump is long. Anyway, transternal closure of the bronchial stump does not deal with the always associated pleural empyema, which will deserve specific treatment. Thus, as described above, chest wall surgery, namely open window thoracostomy (Figure 1A,B) and/or thoracoplasty (Figure 1C) are often the only effective treatments but they maybe psychologically disabling for the patient. In particular, the open window thoracostomy is usually poorly tolerated by patients that must be undergone to daily medications by gauzes for a long period. It is largely accepted that iterative surgery (either re-suturing or open window or thoracoplasty) is required in case of large BPFs with many authors strongly recommending to operate on when BPF diameter is more than 8 mm (13).
At the time of BPF diagnosis, many patients have very poor clinical conditions and the choice of a more conservative than surgical treatment is often influenced by this aspect. In other words, patients with delayed diagnosis of BPF are usually debilitated by long-standing infection and they are not fit to tolerate the stress of a second surgical intervention. For these reasons, many endoscopic therapeutic options have been attempted to close the fistula, but, to date, good results are almost anecdotically reported and seems to be fortuitous more than supported by a robust strategy of care. Anyway, closing BPF does not means healing of empyema, which will deserve specific treatment, although this one will be easier because of stopping of continuous contamination of pleural space by airways germs.
Several endoscopic procedures that have been proposed to close a BPF, including the application of glues, sclerosant agents, plugs placement and endoscopic prosthesis. The application of different products such as fibrin glue, absolute ethanol injection, silver nitrate, spongy calf bone, coils, surgical sponges, acrylic glues are used for smaller lesions. The results seem to be effective for small BPF with the highest success rate for fistulas of 1–3 mm in diameter. The applications of these produces are generally well tolerated because they can be generally administered by flexible fiberoptic bronchoscopy through a conscious sedation (5).
In case of larger defects (more than 5 mm), glues or fibrin applications are usually not effective and the association of plugs achieves better results. A number of cases report are published about the application of different kind of plugs such as demineralized human donor spongiosa, fibrin glue-coated collagen patch, oxidized regenerated cellulose (5,14). Despite the few supporting data, we may assume that this approach could be really appropriate for BPFs less than 5 mm only.
Large defects appear to be particularly challenging and their endoscopic management to perform a bronchial closure with small devices (i.e. absorbable plugs or glues) is very often ineffective because of the poor stability of the device in the defect and considering the risk of spill over the device into the pleural space.
Several reports have been recently published on the use of new devices such as Amplatzer double-disk occludes to manage both large and small BPFs that originate in the main bronchi and lobar bronchi, respectively (15,16). Amplatzer endovascular devices have traditionally been used by cardiologists to close congenital atrial septal defects; it is a device made by two self-expandable disk of nitinol web designed to provide effective closure of the defect by expanding themselves. While its main function stays in inducing an endothelial response in the endovascular use, when placed endoscopically in the bronchial tree, the device may induce a local formation of granulation tissue able to reinforce the mechanical closure of bronchial defect. Endobronchial Amplatzer placement requires general anaesthesia and such procedures is usually performed through an endotracheal tube with flexible bronchoscopy or with rigid bronchoscopy. Even if Amplatzer was well tolerated by the patients, no large studies have been conducted in this setting and few potentially fatal complications are reported in literature due to the erosive effect of disks on bronchial and vascular tissues (17). Another life-treating event is represented by a massive bleeding for erosion of lung vessels already weakened by pleural infection (18). Accordingly, we should be very cautious in the adoption of Amplatzer devices, especially when the fistula is located near to major vascular structures and in presence of a “frail” bronchial tissue.
As an alternative to this approach, the use of airway stents in the management of postpneumonectomy bronchopleural fistulas has also been reported both as a definitive treatment and as a “bridge treatment” to allow an adequate improvement of the patients’ clinical status to completion/definitive surgical attempt (5).
There are only very few articles in the literature reporting the use of stents in cases with a post-lobectomy or post-pneumonectomy bronchopleural fistulas (19-21). Dumon silicone stent was largely adopted in late 90s’ (19), usually placed through a rigid bronchoscope under general anesthesia, with some positive results (20,21). Dumon’s stent are preferable to other device for its tendency to have a low rate of tissue proliferation and ingrowth of granulation tissue. On the other hand, one of the most frequent complication with this silicone stent is the migration of the stent, this representing the main failure of this method, especially in patients with post-pneumonectomy fistula due to the different caliper between tracheal and bronchial lumens. Since 2005 the use of covered self-expandable metallic stents has been reported with or without customized metallic stent (Figure 2). The larger series reported encouraging results with a low rate of stent migration (22-24).
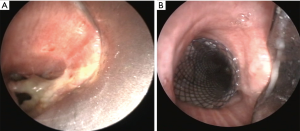
Generally, in all studies in which covered self-expandable metallic stents were used the rate of success is almost high (between 80% and 100%). In this context, the experience reported by Han et al. (25) on the use of customized covered self-expandable metallic stents in 148 patients deserves greater attention by the physicians involved in this field. The authors reported a successful repair of bronchial defect in 96.6% of cases after the first attempt. Such good results are clearly correlated to the large expertise of this Team and in detail to the appropriate selection of the best candidates to this approach. In these cases, the use of self-expandable metallic stent can effectively achieve a total exclusion of the selected bronchial tree and, accordingly, reduce the volume of the pleural cavity. Nevertheless, the mortality rate remains high even in experienced centers and it may be attributable to the sepsis in the majority of cases. Therefore, it is logical to suppose that an early recognizing and treatment of BPF represents the main factor influencing the prognosis of these patients, because the resolution of air leakage has a direct positive effect on mortality. This can explain the optimal result (in terms of early/long-term mortality) of some experiences in which patients with BPFs were treated early and before the onset of pleural contamination (22).
By comparing the early results after endoscopic stenting, we observe that post-lobectomy BPF has the better rate of resolution when compared to post-pneumonectomy fistula probably due to the limited volume of the pleural cavity that accelerate the healing of fistula. As reported above, the timing of BPF onset represents another relevant factor influencing the result after stent placement. Early onset fistula has a better chance of resolution than intermediate and late onset BPFs. Analysing the study of Han et al. (25), the stent was removed earlier in early BPF than other ones, this supporting the logical assumption that BPFs with intermediate or late onset presented several factors (related to patients’ clinical status or related to local anatomical troubles) that obstacle the resolution of the bronchial defect.
Moreover, the adequate adherence of stent to the tracheal/bronchial wall is a crucial point in this challenging management. Customized stents may give higher adherence to the bronchial wall and may reduce the risk of migration; the conical shape is particularly useful for stenting airways with decreasing diameters from the trachea to the left- or right-main-stem bronchus. The “I-shape” self-expandable hinged covered metallic stent with a bullet head was used for BPFs with bronchial stump length more than 20 mm while the “Y-shaped” self-expandable stent was usually adopted when the length of bronchial stump was 5–20 mm. Lastly, in case of short bronchial stump (length <5 mm), the “L-shaped” self-expandable covered metallic stent was largely preferred to the other ones. For not customized stents, the oversizing of the stent may ensure a maximal opposition and thanks also to the use of titanium helical tacks it may prevent the stent migration (22-24).
Regardless by the type of endobronchial metallic stent, expectoration difficulties and sputum retention are often reported and may represent a trigger for lung infection; chest discomfort or pain are also described (25) but one the main feared complication is the abnormal growth of granulation tissue as a consequence of stent placement. This even occurs in about 2/3 of patients with different levels of severity [101/148 patients in the Han’s experience (25)]; the granulation tissue usually growth in the caudal border of endobronchial stent causing in the worst scenarios an airway stenosis with various grade of breathing difficulty; in these cases, the cauterization of granulation tissue and stent removal are usually needed, as performed in 6 patients of the series described by Han and co-workers (25).
Therefore, considering that BPF represents one of the main challenges for endoscopists and thoracic surgeons, the strategy of care should be carefully set in a multidisciplinary framework and in high-volume centers with expertise in this field. To summarize the main take home messages, the physicians should take firmly in mind that the effectiveness of treatments and mortality are strictly dependent from (I) type of fistula, (II) time of onset, (III) early recognizing and (VI) baseline clinical condition of the patient.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 2006;81:1013-9; discussion 1019-20. [Crossref] [PubMed]
- Cerfolio RJ. The incidence, etiology, and prevention of postresectional bronchopleural fistula. Semin Thorac Cardiovasc Surg 2001;13:3-7. [Crossref] [PubMed]
- Sirbu H, Busch T, Aleksic I, et al. Bronchopleural fistula in the surgery of non-small cell lung cancer: incidence, risk factors, and management. Ann Thorac Cardiovasc Surg 2001;7:330-6. [PubMed]
- Asamura H, Naruke T, Tsuchiya R, et al. Bronchopleural fistulas associated with lung cancer operations. Univariate and multivariate analysis of risk factors, management, and outcome. J Thorac Cardiovasc Surg 1992;104:1456-64. [PubMed]
- Lois M, Noppen M. Bronchopleural fistulas. An overview of the problem with a special focus on endoscopic management. Chest 2005;128:3955-65. [Crossref] [PubMed]
- Wright CD, Wain JC, Mathisen DJ, et al. Postpneumonectomy bronchopleural fistula after sutured bronchial closure: incidence, risk factors, and management. J Thorac Cardiovasc Surg 1996;112:1367-71. [Crossref] [PubMed]
- Shapiro M, Swanson SJ, Wright CD, et al. Predictors of major morbidity and mortality after pneumonectomy utilizing the society for thoracic surgeons general thoracic surgery database. Ann Thorac Surg 2010;90:927-34; discussion 934-5. [Crossref] [PubMed]
- Zanotti G, Mitchell JD. Bronchopleural Fistula and Empyema After Anatomic Lung Resection. Thorac Surg Clin 2015;25:421-7. [Crossref] [PubMed]
- Di Maio M, Perrone F, Deschamps C, et al. A meta-analysis of the impact of bronchial stump coverage on the risk of bronchopleural fistula after pneumonectomy. Eur J Cardiothorac Surg 2015;48:196-200. [Crossref] [PubMed]
- Ponn RB. Complications of Pulmonary Resection. In: Shields TW, Locicero J 3rd, Ponn RB, et al. editors. General Thoracic Surgery. 6th ed. Philadelphia, PA: Lippincott Williams and Wilkins, 2005:554-86.
- Le Brigand H. Fistules bronchiques après pneumonectomies. In: Le Brigand H (ed). Appareil respiratoire, mediastin, paroi thoracique. Paris: Ed Masson, 1973:462-70.
- Stamatis G, Martini G, Freitag L, et al. Transsternal transpericardial operations in the treatment of bronchopleural fistulas after pneumonectomy. Eur J Cardiothorac Surg 1996;10:83-6. [Crossref] [PubMed]
- Bribriesco A, Patterson GA. Management of Postpneumonectomy Bronchopleural Fistula. Thorac Surg Clin 2018;28:323-35. [Crossref] [PubMed]
- Fiorelli A, Frongillo E, Santini M. Bronchopleural fistula closed with cellulose patch and fibrin glue. Asian Cardiovasc Thorac Ann 2015;23:880-3. [Crossref] [PubMed]
- Fruchter O, Kramer MR, Dagan T, et al. Endobronchial closure of bronchopleural fistulae using Amplatzer devices: our experience and literature review. Chest 2011;139:682-7. [Crossref] [PubMed]
- Fruchter O, El Raouf BA, Abdel-Rahman N, et al. Efficacy of bronchoscopic closure of a bronchopleural fistula with amplatzer devices: long-term follow-up. Respiration 2014;87:227-33. [Crossref] [PubMed]
- DiBardino DJ, McElhinney DB, Kaza AK, et al. Analysis of the US Food and Drug Administration Manufacturer and User Facility Device Experience database for adverse events involving Amplatzer septal occluder devices and comparison with the Society of Thoracic Surgery congenital cardiac surgery database. J Thorac Cardiovasc Surg 2009;137:1334-41. [Crossref] [PubMed]
- Buitrago DH, Pinto D, Berkowitz SJ, et al. Fatal Hemoptysis After Closure of Gastrobronchial Fistula Using an Amplatzer Vascular Device. Ann Thorac Surg 2018;105:e71-3. [Crossref] [PubMed]
- Tsukada H, Osada H. Used of a modified Dumon stent for postoperative bronchopleural fistula. Ann Thorac Surg 2005;80:1928-30. [Crossref] [PubMed]
- Watanabe S, Shimokawa S, Yotsumoto G, et al. The use of a Dumon stent for the treatment of a bronchopleural fistula. Ann Thorac Surg 2001;72:276-8. [Crossref] [PubMed]
- Cusumano G, Terminella A, Vasta I, et al. Endoscopic stenting for double bronco-pleural fistula after lobectomy. Asian Cardiovasc Thorac Ann 2015;23:995-7. [Crossref] [PubMed]
- Andreetti C, D'Andrilli A, Ibrahim M, et al. Submucosal injection of the silver-human albumin complex for the treatment of bronchopleural fistula. Eur J Cardiothorac Surg 2010;37:40-3. [Crossref] [PubMed]
- Dutau H, Breen DP, Gomez C, et al. The integrated place of tracheobronchial stents in the multidisciplinary management of large postpneumonectomy fistulas: our experience using a novel customised conical self-expandable metallic stent. Eur J Cardiothorac Surg 2011;39:185-9. [Crossref] [PubMed]
- Cao M, Zhu Q, Wang W, et al. Clinical Application of Fully Covered Self-Expandable Metal Stents in the Treatment of Bronchial Fistula. Thorac Cardiovasc Surg 2016;64:533-9. [Crossref] [PubMed]
- Han X, Yin M, Li L, et al. Customized airway stenting for bronchopleural fistula after pulmonary resection by interventional technique: single-center study of 148 consecutive patients. Surg Endosc 2018;32:4116-24. [Crossref] [PubMed]


