The normative value of inflammatory cells in the nasal perfusate of Chinese adults: a pilot study
Introduction
The nasal mucosa is the portion of the airway mucosa that might be the most convenient tissue for examining the effects of mucosal inflammation. Since Hansel first reported the examination method (1) in 1934, increasing numbers of researchers have performed studies on the effects of allergies, blood vessel activity and infection on rhinitis in nasal cells (2,3). The analysis of nasal cells can reveal alterations in the epithelial cells after exposure to physical and chemical inflammatory factors (4) and the progression of acute and chronic inflammation (5), which is of great interest in basic and clinical research. This type of examination can be easily repeated and used in patients of different ages due to its broad approach, simplicity and non-invasiveness (6). However, rhino cytology is only used in basic and subclinical research and is not effective in clinical applications (7). Although the examination is simple and recommended by experts, there is no consensus on the standards for rhino cytology, especially for cell counting (8), which is usually applied to rhinitis patients (9) but not to healthy people (10). There are few reports on the normative values for the rhino cytology of nasal perfusates, but determining the classification for mucosal inflammation, categorizing the inflammatory status, updating the progression of inflammation and evaluating the treatment of inflammation are important (11). In addition, discussing the relevance of this analysis for lower airway inflammation has great clinical significance (12). The common rhino cytological examination consists of a smear of nasal secretion, nasal lavage, nasal brush and biopsy of the nasal mucosa. Inflammatory cells are highly relevant to various examinations (13). Although scraping samples are better than lavage samples, lavage is still the most stable and accurate examination for assessing the inflammatory status of the nose (3), and the repeatability of NAL is sufficient (14). Our research first focused on the nasal lavage of 500 healthy individuals and established a convenient, stable lavage and counting method for rhino cytology. We also determined the normal values for inflammatory cells in nasal lavage, which could provide clues for further research on the relevance between nasal and lower airway inflammatory diseases.
Subjects and methods
Subjects
A total of 500 healthy individuals treated at Nanjing Jinling Hospital and Guangzhou Respiratory Institute for Ordinary Physical Examination were enrolled in this study. The inclusion criteria were as follows: (I) negative result in the skin prick test; (II) all CBC values were normal; (III) non-smokers aged from 16-60 years old (250 cases from 16-30 years old and 250 cases from 31-60 years old with a male-to-female ratio of 1:1); (IV) no chronic respiratory diseases, no allergic constitution, no digestive difficulties and no history of other severe diseases; (V) no nasal spray, antihistamine, glucocorticoid or LTR treatment in the last week and (VI) no respiratory infection within the last four weeks. This study was approved by the Ethics Committee of Nanjing Jinling Hospital and Guangzhou Respiratory Institute. All of the participants were fully informed about the purpose of this study. All participants provided written informed consent before participation in the study.
Methods
Based on studies performed abroad (15,16), we performed the procedures as follows. First, we allowed the patients to sit with their heads anteverted at 45 degrees. Second, we told the patients to breathe through their mouths to close the nasopharynx and prevent the irrigation from flooding into the pharynx oralis. Third, we irrigated one of the nostrils with 5 mL of 37 °C saline usina syringe and closed the nostril with a plug to thoroughly irrigate the middle and lower nasal passages. The irrigation fluid was collected with a funnel (saline spilling from the other nostril is a sign of thorough irrigation). The irrigation was repeated three times, and the total time for the irrigation was approximately 5 minutes. Some of the fluid was removed by suction, and the remainder was collected with a funnel. Fourth, we performed the same procedure in the other nostril. Fifth, we recorded the volume and collection rate. Sixth, we stored the sample at 4 °C and ran the tests within 2 hours.
Apparatus and reagents
A centrifugal machine, microscope, oscillator, water bath, pipettor, electronic balance, hematoxylin-eosin dye solution (Nanjing Jiancheng Technology Company; Serial number: D006) and DTT (dispensed with Amresco, purity >99%) were utilized in this study.
Criterion of acceptability
A recollection rate of higher than 70% was acceptable.
Cellular test
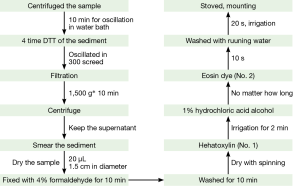
The irrigated sample was collected and centrifuged. The supernatant was stored (used for other related data), and the sediment was used for cellular testing (Figure 1).
Cell counting
We observed the slice under a light microscope at 200 times magnification and counted the total number of various inflammatory cells, including eosinophils (Eos), neutrophils (N), macrophages (M) and lymphocytes (L), in five fields and identified every cell type at 200 HP. The total number of inflammatory cells indicated the severity of rhinitis.
Statistic analysis
In this study, the data are all given as the and were analyzed using SPSS (version 18.0). The Kolmogorov-Smirnoff test was used to test the normality of the distribution, and the reference range was shown to be 95%. The Mann-Whitney U non-parametric test was used for the analysis between the male and female participants and among the different age groups. A P value less than 0.05 indicated a significant difference.
Results
Consistency test
There were no significant differences in the age, sex or education level of the patients between the two hospitals (χ2=2.33, P>0.05; χ2=2.92, P>0.05; χ2=3.01, P>0.05), indicating the consistency of the data.
Establishment of a method for nasal lavage
Nasal lavage samples were collected from 500 individuals. Cough occurred in 9 cases due to unconscious aspiration during irrigation (1.80%), and there was a collection rate of less than 70% in 12 cases (2.40%). Finally, we included a total of 479 cases (95.80%).
The normal range of cells in the nasal lavage
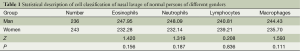
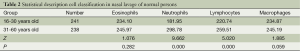
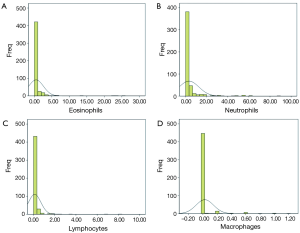
The numbers of all inflammatory cells showed a skewed distribution. The median number and interquartile range (IQR) of the Eos were 0 and 0.2, respectively. These values were 0.4 and 2.2, respectively, for N and 0 and 0, respectively, for both L and M. There was no significant difference between male and female patients (Table 1). There were significant differences for the N and L among the different age groups (P=0.000), but there were no differences for the Eos and M (P>0.05) (Table 2). The 95% UULVs of the Eos and N in the nasal perfusate were 2.99 and 14.94, respectively, for group A and 1.41 and 17.08, respectively, for group B. As a result, the total 95% UULVs of the Eos and N in the nasal perfusate were 2.00 and 16.80, respectively (Tables 1-3 and Figures 2-4).
Full table
Full table

Full table
Discussion
Nasal lavage is a non-invasive method that is easily standardized and highly repeatable. Meanwhile, tests of the supernatant from the centrifuged lavage fluid could indicate various inflammatory conditions (17). Therefore, a standardized method and normal range of rhino cytology are important for research on the nasal passages and lower respiratory tract. We performed research on 500 individuals and primarily confirmed the normal range of nasal cells in Chinese people.
We first established the cell counting method for the nasal lavage of healthy Chinese people and determined the normative range. Although there were some reports on noses with nasal lavage (18), they mainly focused on rhinitis and the normative range for Eos but not on the normal range of N, L, M and other nasal cells (3,19), and the methods differed among the reports (e.g., nasal brushing, scraping, smearing and lavage). The counting methods and normative ranges also differed. However, no research of this type has been performed in China. As a result, determining the normal range of each type of inflammatory cell in the nasal lavage was necessary. The counting range differed by researcher, and nearly all researchers in this study counted the Eos, N and L cells. They also advised that rhinitis should be studied based on all of the nasal cell types, such as goblet cells, M and epithelial cells (columnar and squamous) (19). With repeated studies, we finally confirmed that the nasal lavage could be evaluated using Eos, N, L and M and that other cells should be excluded. First, abnormally dyed cells (basophils and mast cells) were detected in only a few cases (15/1,620), and such findings had no significance for further data analysis (19) based on the smears of nasal secretions (relevance ratio: 0.93%). Second, a previous study found no abnormally dyed cells, most likely because the preparation of the sample from collection to processing was more complicated than a nasal smear, which might result in the destruction of the cells. Third, abnormally dyed cells were all immediate phase cells that could secrete inflammatory molecules, such as histamine and tachykinin, which could destroy the cells at an early stage (3). Fourth, rhinitis, especially AR, was represented by Eos, which are the core marker cell. The N cells are closely related to nasal infectious diseases, and the L cells are most likely associated with allergies. M are related to atmospheric contamination, such as glass fibers in the air and ozone (16,20). Fifth, epithelial cells are the basic structure of the nasal mucosa and do not reflect the severity of rhinitis. Sixth, Eos, N, L and M cells were commonly observed in the induced sputum (21), providing a foundation for the investigation of the relevance of upper and lower airway disease (22).
Some reports have indicated that the severity of rhinitis correlates with the percentage of each inflammatory cell type (8). There is a significant correlation between nasal Eos and nasal symptoms according to various data from nasal lavages (IL-4, IL-5, IL-8 and IFN-γ), nasal resistance and pulmonary function. The correlation index is as high as 0.95, i.e., Eos play an important role in nasal cell tests (23). The sensitivity and specificity vary among the nasal tests that are performed abroad, mainly due to the differences in sampling, counting and scoring (8). For example, Mygind et al. (24) suggested that it should be 10%, whereas Jankowski et al. (25) indicated that it should be 20%; however, Burrows et al. (26) reported that it should be 25%. A recent study showed that more than 8% of Eos could be the standard for allergic rhinitis (AR) in children under four years old (sensitivity: 80%; specificity: 95%) (19). However, these so-called standards were not based on research performed on healthy individuals.
The most popular field for research on rhinitis is AR. Basophils and mast cells are commonly the main course for the immediate phase of histamine release during the early stage of AR, and Eos and M can release the associated inflammatory molecules (18), which are related to the symptoms during the delayed phase. However, little is known about the effects of N and M in rhinitis. In 2004, Fransson et al. (27) reported on the role of N in intermittent AR and stated that N cells not only led to early-stage reactions but also are strongly associated with the symptoms of rhinitis, suggesting that N cells are the motor for the secretion of AR-related molecules in the early stage. However, this work has not been supported by further study. We found that the average number of N cells was 10 times higher than that of Eos (95% UULV of 16.80) and was far higher than the number of Eos in the nose, which might have occurred because CO2 and ozone can elevate the N chemo attractive factor (28). Both the absolute numbers and 95% UULVs of L and M cells were low, especially for the M cells. There was only one cell in 25 slides. Research on L and M cells has been nearly absent. Recently, some studies have indicated that there are different nasal cells involved in the different types of rhinitis. L and M cells are much more common in patients with medium-severe, constant AR (29). A high concentration of ozone could decrease the number of M cells (20). Limited research has provided us clues for further studying and detailing the treatment of rhinitis.
Research from animal models and human subjects suggests that there are several important changes in the innate and adaptive immune responses with increasing age, a phenomenon termed ‘‘immunosenescence’’ (30). The function of immune-associated inflammatory cells, including the number, function and early-stage reaction, decreased in the older population, when activated (31). However, there are currently few reports on the age-associated changes in the inflammatory cell count and function. As a result, we divided the subjects into two groups, group A (16-30 years old) and group B (31-60 years old), and the average rank in group B was higher than that in group A. There were no significant differences in the Eos and M (Table 2), although the P value was close to 0.05 for the M. In contrast, there were significant differences in the N and L (P=0.000). A comparison of another in vitro Eos effect or function, leukotriene C4 production, revealed no difference between older and younger asthmatic subjects (32). Mathur et al. reported that there was also no significant difference between younger and older age groups in either the percentage or absolute number of Eos in the induced sputum of asthma patients. However, the degradation of Eos after stimulation with IL-5 was slower in the older group, and the production of ECP after stimulation was also slower. Therefore, age most likely affects the activity of Eos (21) but does not affect the absolute number of Eos. In addition to Eos, we also found that the N, Land M cells were all higher in the younger group, which is in agreement with the findings of other studies. As people age, their immune function declines, and they acquire infections more easily (21,33). Hence, we should pay more attention to age in our study.
Although we collected a large number of samples and studied the normal values in detail, there are still some disadvantages in this study. First, the concentration of inflammatory cells was not determined. There should be a primary result for the normal values of the concentrations of various inflammatory cells in the nasal lavage. Second, some of the study components used repeated nasal sprays and collected the condensed water from the anterior naris before analyzing the result. This method is more acceptable in children, which will be applied in a future study (27). Third, we did not collect samples from individuals with rhinitis. If there are data on AR, we can obtain the optimum value for diagnosing AR from the ROC (34) and determining the effect of AR on nasal cytology.
Conclusions
In conclusion, we developed a method for obtaining nasal lavages and determined the nasal cytology of healthy adults based on research conducted in two centers. We will further perform multi-center research to validate this study and prove the usefulness of these measures.
Acknowledgements
This work was supported by the grant of Open Research of State Key Laboratory of Respiratory Diseases (2007DA780154F0907) and National Science Fund Committee (NSFC81072366). We thank Zhihao Liu from the Jiangsu Provincial Center for Disease Prevention and Control for support with the statistical analysis.
Disclosure: The authors declare no conflict of interest.
References
- Matheson A, Rosenblum A, Glazer R, et al. Local tissue and blood eosinophils in newborn infants. J Pediatr 1957;51:502-9. [PubMed]
- Ventura MT, Gelardi M, D’Amato A, et al. Clinical and cytologic characteristics of allergic rhinitis in elderly patients. Ann Allergy Asthma Immunol 2012;108:141-4. [PubMed]
- Di Lorenzo G, Mansueto P, Pacor ML, et al. Clinical importance of eosinophil count in nasal fluid in patients with allergic and non-allergic rhinitis. Int J Immunopathol Pharmacol 2009;22:1077-87. [PubMed]
- Glück U, Schütz R, Gebbers JO. Cytopathology of the nasal mucosa in chronic exposure to diesel engine emission: a five-year survey of Swiss customs officers. Environ Health Perspect 2003;111:925-9. [PubMed]
- Gelardi M, Fiorella ML, Leo G, et al. Cytology in the diagnosis of rhinosinusitis. Pediatr Allergy Immunol 2007;18 Suppl 18:50-2. [PubMed]
- Gelardi M. Atlas of nasal cytology. Torino: Centro Scientifico Editore, 2004.
- Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008;63 Suppl 86:8-160. [PubMed]
- Canakcioglu S, Tahamiler R, Saritzali G, et al. Evaluation of nasal cytology in subjects with chronic rhinitis: a 7-year study. Am J Otolaryngol 2009;30:312-7. [PubMed]
- Garavello W, Somigliana E, Acaia B, et al. Nasal lavage in pregnant women with seasonal allergic rhinitis: a randomized study. Int Arch Allergy Immunol 2010;151:137-41. [PubMed]
- Sanli A, Aydin S, Ateş G, et al. Comparison of nasal smear eosinophilia with skin prick test positivity in patients with allergic rhinitis. Kulak Burun Bogaz Ihtis Derg 2006;16:60-3. [PubMed]
- Scadding GK, Durham SR, Mirakian R, et al. BSACI guidelines for the management of rhinosinusitis and nasal polyposis. Clin Exp Allergy 2008;38:260-75. [PubMed]
- Gelardi M, Passalacqua G, Fiorella ML, et al. Nasal cytology: the “infectious spot”, an expression of a morphological-chromatic biofilm. Eur J Clin Microbiol Infect Dis 2011;30:1105-9. [PubMed]
- Piacentini GL, Kaulbach H, Scott T, et al. Evaluation of nasal cytology: a comparison between methods. Allergy 1998;53:326-8. [PubMed]
- Castano R, Thériault G, Maghni K, et al. Reproducibility of nasal lavage in the context of the inhalation challenge investigation of occupational rhinitis. Am J Rhinol 2008;22:271-5. [PubMed]
- Schiavino D, Nucera E, Milani A, et al. Nasal lavage cytometry in the diagnosis of nonallergic rhinitis with eosinophilia syndrome (NARES). Allergy Asthma Proc 1997;18:363-6. [PubMed]
- Paananen H, Holopainen M, Kalliokoski P, et al. Evaluation of exposure to man-made vitreous fibers by nasal lavage. J Occup Environ Hyg 2004;1:82-7. [PubMed]
- Watelet JB, Gevaert P, Holtappels G, et al. Collection of nasal secretions for immunological analysis. Eur Arch Otorhinolaryngol 2004;261:242-6. [PubMed]
- Scavuzzo MC, Rocchi V, Fattori B, et al. Cytokine secretion in nasal mucus of normal subjects and patients with allergic rhinitis. Biomed Pharmacother 2003;57:366-71. [PubMed]
- Nowacki Z, Neuberg J, Strzałka K, et al. Is prediction of the allergic march possible on the basis of nasal cytology? Pneumonol Alergol Pol 2010;78:263-70. [PubMed]
- Liu L, Leech JA, Urch RB, et al. A comparison of biomarkers of ozone exposure in human plasma, nasal lavage, and sputum. Inhal Toxicol 1999;11:657-74. [PubMed]
- Mathur SK, Schwantes EA, Jarjour NN, et al. Age-related changes in eosinophil function in human subjects. Chest 2008;133:412-9. [PubMed]
- Trivedi SG, Lloyd CM. Eosinophils in the pathogenesis of allergic airways disease. Cell Mol Life Sci 2007;64:1269-89. [PubMed]
- Ciprandi G, Vizzaccaro A, Cirillo I, et al. Nasal eosinophils display the best correlation with symptoms, pulmonary function and inflammation in allergic rhinitis. Int Arch Allergy Immunol 2005;136:266-72. [PubMed]
- Mygind N, Nielsen LP, Hoffmann HJ, et al. Mode of action of intranasal corticosteroids. J Allergy Clin Immunol 2001;108:S16-25. [PubMed]
- Jankowski R, Persoons M, Foliguet B, et al. Eosinophil count in nasal secretions of subjects with and without nasal symptoms. Rhinology 2000;38:23-32. [PubMed]
- Burrows B, Hasan FM, Barbee RA, et al. Epidemiologic observations on eosinophilia and its relation to respiratory disorders. Am Rev Respir Dis 1980;122:709-19. [PubMed]
- Fransson M, Benson M, Wennergren G, et al. A role for neutrophils in intermittent allergic rhinitis. Acta Otolaryngol 2004;124:616-20. [PubMed]
- Holgate ST, Sandström T, Frew AJ, et al. Health effects of acute exposure to air pollution. Part I: Healthy and asthmatic subjects exposed to diesel exhaust. Res Rep Health Eff Inst 2003:1-30; discussion 51-67. [PubMed]
- Gelardi M, Incorvaia C, Fiorella ML, et al. The clinical stage of allergic rhinitis is correlated to inflammation as detected by nasal cytology. Inflamm Allergy Drug Targets 2011;10:472-6. [PubMed]
- Busse PJ, Mathur SK. Age-related changes in immune function: effect on airway inflammation. J Allergy Clin Immunol 2010;126:690-9. [PubMed]
- Gomez CR, Nomellini V, Faunce DE, et al. Innate immunity and aging. Exp Gerontol 2008;43:718-28. [PubMed]
- Nyenhuis SM, Schwantes EA, Mathur SK. Characterization of leukotrienes in a pilot study of older asthma subjects. Immun Ageing 2010;7:8. [PubMed]
- Plackett TP, Boehmer ED, Faunce DE, et al. Aging and innate immune cells. J Leukoc Biol 2004;76:291-9. [PubMed]
- Khianey R, Oppenheimer J. Is nasal saline irrigation all it is cracked up to be? Ann Allergy Asthma Immunol 2012;109:20-8. [PubMed]