Clinicopathological variables predicting HER-2 gene status in immunohistochemistry-equivocal (2+) invasive breast cancer
Background
Breast cancer is regarded as a heterogeneous group of tumors that are diverse in terms of underlying biology, pathological characteristics, response to therapy, and clinical outcome (1). Breast cancer is divided into at least five distinct molecular subtypes [luminal A, luminal B, human epidermal growth factor receptor-2 (HER-2), normal-like, and basal] by gene expression analysis (2). Breast cancer with HER-2 overexpression currently comprises 15% to 20% of all cases in the world (3). HER-2/neu, located on chromosome 17q21, encodes for the 185 kD transmembrane glycoprotein HER-2, which is one of the most targeted proteins. Studies indicate that HER-2 is involved in the activation of intracellular signal transduction pathways that regulate cell growth, proliferation, adhesion, and motility (4). HER-2overexpression or amplification in breast cancer has been extensively studied worldwide (5-7). Overexpression or amplification of HER-2 has been demonstrated to be an independent parameter for bad prognosis, and is shown to be associated with resistance to certain chemotherapeutic agents (8-11). HER-2-targeted therapies have significantly improved disease-free survival in women with HER-2-positive cancers both in early and metastatic breast cancer (12,13). Three HER-2-targeted agents, trastuzumab (Herceptin), lapatinib (Tykerb), and pertuzumab (Perjeta), have been made available in the past decade for the treatment of HER-2-positive metastatic breast cancer (14). Combinations of HER-2-directed agents may yield additive or synergistic effects that lead to better prognosis (15).
Overexpression of the HER-2 protein has become a marker for eligibility for HER-2-directed treatments. False positive or false negative results in HER-2 patients may lead to inappropriate treatment administration (16). Therefore, HER-2 status is crucial in the guidance of treatment decisions for the use of trastuzumab and is becoming a standard recommendation in the pretreatment work-up of patients with invasive breast cancer. Two conventional methods are used for determining HER-2 status, namely, immunohistochemistry (IHC) and fluorescent in situ hybridization (FISH). IHC is most frequently used in initial pathological tests for HER-2 protein expression, and is convenient and inexpensive. HER-2 IHC results are generally divided into four scale scores (range, 0-3+) on the basis of percentage of positive tumor cells and staining intensity. The US Food and Drug Administration (FDA) and American Society of Clinical Oncology/College of American Pathologists (ASCO/CAPs) recommends that HER-2 IHC scores of 0 and 1+ should be regarded as HER-2 negative and those with HER-2 3+ scores should be considered HER-2 positive. An invasive breast cancer with HER-2 2+ score is regarded as HER-2 equivocal and should be further assessed by FISH, which is considered the standard test for HER-2 status. FISH is more accurate and reliable than IHC; however, its use for routine testing is hindered by drawbacks such as high cost, need for a skilled operator, long procedure, need for special equipment, and difficult preservation of slides for later review.
Invasive breast cancer with HER-2 2+ IHC status can be divided into two groups: those that have been possibly HER-2 amplified and those that have not been HER-2 amplified. Going et al. (17) interpreted 4,343 assessable HercepTests on successive breast cancer tissues and found that 35.7% (315/883) of patients with HER-2 2+ were HER-2 amplified. A few studies have reported the possibility of predicting HER-2 positivity from HER-2 2+ IHC samples (18,19). In our present study, we designed a retrospective clinical analysis to develop a multivariate logistic regression analysis that predicts the presence of HER-2 amplification in HER-2 2+ invasive breast cancer patients.
Materials and methods
Patients
The present study enlisted 277 operable patients diagnosed with invasive breast cancer between October 2006 and December 2012 at Zhejiang Cancer Hospital, China. All patients were newly confirmed for invasive breast cancer status and have not received treatment. A total of 182 patients with HER-2 2+ IHC evaluation were included in this study. The extent of disease was determined by TNM staging according to the new staging system of the American Joint Committee on Cancer/International Union against Cancer (AJCC/UICC) (20). Patient clinical history and tumor characteristics were obtained from histopathology reports and medical records. Gathered data included patient age, tumor location, histological grade, tumor size, regional lymph node status, lympho-vascular invasion (LVI), estrogen receptor (ER), progesterone receptor (PR), HER-2 status, and Ki-67 index. This study was approved by the Institutional Review Board of the hospital. All patients provided informed consent prior to surgery.
Immunohistochemistry (IHC)
All surgical specimens were routinely fixed in 10% buffered formalin solution and embedded in paraffin. Each specimen was verified by two pathologists before inclusion in this study. HER-2 IHC was performed on unstained sections from representative paraffin blocks using HercepTest. After deparaffinization and dehydration, tissue sections were placed in 0.1 M sodium citrate buffer (PH 6) for 40 min at 99 °C, after which the antigen was retrieved. The slides were cooled at room temperature, rinsed with distilled water, incubated with rabbit monoclonal anti-human HER-2/neu antibody for 1 h, then applied with biotinylated secondary antibody for 10 min. The signal was visualized using avidin-peroxidase. The slides were counterstained with Mayer’s hematoxylin solution, dehydrated, and mounted. HER-2 positivity was defined by membranous staining.
HER-2 immunoreactivity was localized in the cell membrane. HER-2 expression was scored using HercepTest according to manufacturer’s recommendations. Guidelines for scoring were as follows: 0, no immunostaining; 1+, faint perceptible staining of the tumor cell membranes; 2+, weak to moderate complete membrane staining in more than 10% of the tumor cells; and 3+, strong circumferential staining of the entire tumor cell membrane.
All cases also underwent ER, PR, and proliferation index (Ki67) IHC testing. A cut-off level of 10% or greater was defined as positive for ER and PR expression. Positivity for Ki67 was defined by a cut-off level of 15% or greater.
Fluorescence in situ hybridization (FISH)
HER-2/neu FISH were assessed on all specimens with HER-2 IHC 2+. The selected paraffin-embedded tissues sections (4 µm) containing representative invasive breast cancer cells were analyzed by dual-color FISH (a mixture of a spectrum orange DNA probe, covering a 218 kb region that includes the HER-2 gene, and a spectrum green probe for the chromosome 17 centromere) using the PathVysion HER-2 DNA Probe kit (Vysis, Inc., USA) according to the manufacturer’s instructions. After 5 min denaturation at 82 °C, the slides and probe mix were incubated overnight at 45 °C in a humidified hybridization chamber. The following morning, a fluorescence-mounting medium containing DAPI was applied after a series of stringent washes. The FISH specimens were analyzed on a Nikon Eclipse 80i fluorescence microscope with special filters.
The screening protocol included two independent observers. For each specimen, orange and green signals were counted from a minimum of 80 tumor cell nuclei in at least two distinct areas. HER-2 gene status was evaluated based on the ratio of HER-2 signals and chromosome 17 centromic signals. In our study, a case was regarded HER-2 gene amplified if the ratio of HER-2/CEP17 was equal to or more than 2.0 as FDA recommendation. Also, the result were classified following 2013 ASCO/CAP guideline: positive (HER-2/CEP7 ratio ≥2.0 with an average HER-2 copy number ≥4.0 signals per cell; HER-2/CEP7 ratio ≥2.0 with an average HER-2 copy number <4.0 signals per cell; HER-2/CEP7 ratio <2.0 with an average HER-2 copy number ≥6.0 signals per cell.), equivocal (HER-2/CEP7 ratio <2.0 with an average HER-2 copy number ≥4.0 and <6.0 signals per cell.) and negative (HER-2/CEP7 ratio <2.0 with an average HER-2 copy number <4.0 signals per cell).
Statistical analysis
Pearson’s chi-square test was performed to evaluate the association between clinicopathological variables and HER-2 FISH positivity. Student’s t-test was used to compare the Ki67 between the HER-2 negative and positive group. Risk factors influencing HER-2 FISH positivity were evaluated by unconditional logistic regression analysis. All statistical calculations were performed with SPSS 13.0 for Windows (Chicago, IL, USA). A P value <0.05 was considered statistically significant.
Results
This study included 182 invasive breast cancer patients with HER-2 IHC score of 2+. The characteristics of these patients are summarized in Table 1. The study population had a median age of 48 years (range, 29-78 years). Tumor cell grade was available in 153 patients (84.1%), 105 being grade 1 or 2 (57.7%) and 48 being grade 3 (26.4%). Hormone receptor (HR) status was available in all patients. ER was expressed in 131 (72.0%) patients. PR positivity was shown in 73.1% of the patients (133/182). Median Ki67 value was 20% (range, 3-90%). A total of 121 patients had high and 61 had low Ki67 value, according to the Ki67 cut-off value of 15%. According to the new TNM staging system, 19 of all the cases (10.4%) were stage I, 132 (72.5%) were stage II, and 31 (17.0%) were stage III.
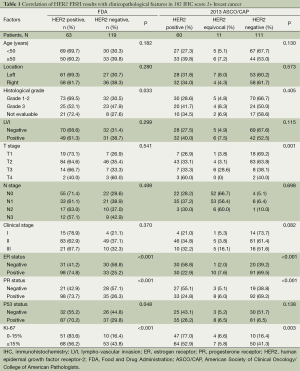
Full table
The distribution of HER-2 FISH results according to both FDA and 2013 ASCO/CAP recommendation are shown in Table 2. HER-2 FISH amplified (positive) was found in 34.6% (63/182) according to FDA criteria and 32.9% (60/182) with 2013 ASCO/CAP guideline. There was good agreement between the FDA and 2013 ASCO/CAP guideline. Some changes have been also observed. There were only three patients who had positive according to FDA criteria that changed to negative according to ASCO/CAP guideline, and five patients with positive based on ASCO/CAP cut-off changed to negative with FDA recommendation. The majority of HER-2 equivocal (ASCO/CAP guideline) patients had HER-2 negative (90.9%, 10/11).
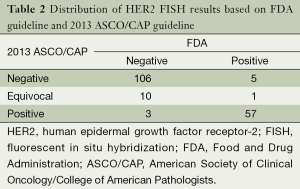
Full table
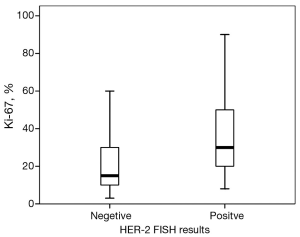
Then, we take the HER-2 test guideline of FDA as the major guideline. Sixty-three of all patients were HER-2 FISH amplified (positive). Patients with HER-2 FISH amplified tumors were more likely to have higher histological grades (χ2=8.73, P=0.033) compared with patients with unamplified tumors. No significant difference between the groups were found with respect to age (<50 vs. ≥50 years, P=0.182), LVI (P=0.299), cancer location (P=0.280), or clinical stage (P=0.370). Tumors with HER-2 amplification were more likely to be ER-negative (58.8% vs. 25.2%, P<0.001), PR-negative (57.1% vs. 26.3%, P<0.001), or P53-negative (44.8% vs. 29.8%, P=0.048). The median percentage of Ki67 was 15% in the non-HER-2-amplified group and 30% in the HER-2-amplified group. A significantly high level of Ki67 was detected in the HER-2-amplified groups (P=0.006, Figure 1). Based on the Ki67 cut-off value of 15%, patients were classified into either of two groups: relatively high Ki67 or low Ki67. A positive correlation was found between Ki67 and HER-2 status (χ2 =13.46, P<0.001).
A logistic regression model was used to reveal risk factors for HER-2 amplification. The association between clinicopathological variables and HER-2 amplification is shown in Table 3. Cases with high Ki67 had significantly higher risk of HER-2 amplification than those with low Ki67 (OR =3.975; 95% CI, 1.846-8.560; P<0.001). Subjects with ER positive expressions were less likely to exhibit HER-2 amplification compared with those with ER negative expression (OR =0.236; 95% CI, 0.119-0.467; P<0.001). The risk was also much reduced in cases with PR positive expressions than those with PR negative expressions (OR =0.268; 95% CI, 0.135-0.531; P<0.001). Subjects with P53 positive expressions were less likely to develop HER-2 amplification (OR =0.523; 95% CI, 0.275-0.997; P=0.049).
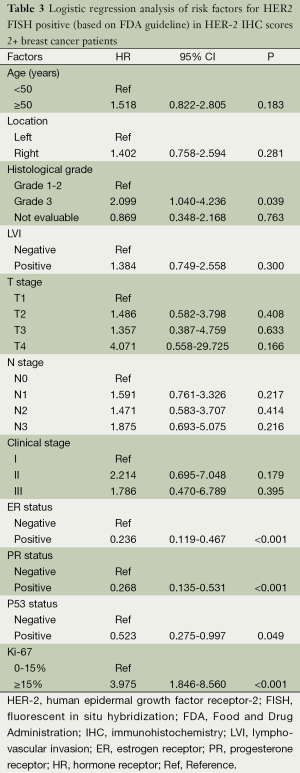
Full table
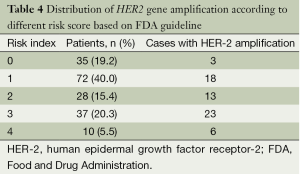
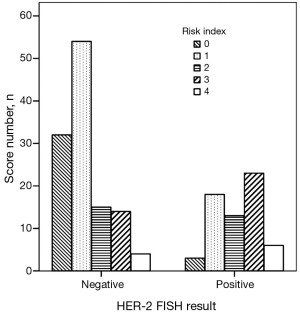
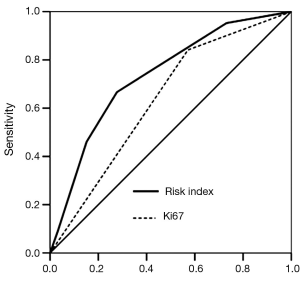
We created a risk score that comprised the following factors: ER (score 1 when IHC negative; 0 when positive), PR (score 1 when IHC negative; 0 when positive), P53 (score 1 when IHC negative; 0 when positive), and Ki67 (score 0 when IHC negative; 1 when positive). The sum of the above parameters allowed the establishment of a risk score for HER-2 FISH amplification (Table 4). A significant association between risk score and HER-2 FISH amplification was observed (χ2=30.41, P<0.001, Figure 2). Receiver operator characteristic curves were constructed to compare the ability of the four tumor markers to differentiate between patients with or without HER-2 FISH amplification. AUC was 0.64±0.04, 0.35±0.05, 0.37±0.05, and 0.43±0.05 for Ki67, ER, PR, and P53. AUC was 0.74±0.04 (95% CI, 0.66-0.81) for the sum of all four markers (Figure 3).
Full table
Discussion
Using trastuzumab supplement for neoadjuvant or adjuvant chemotherapy provides significant survival benefit in invasive breast cancer with HER-2-overexpressing tumor cells. However, for HER-2-negative cases, trastuzumab offers no benefit and only contributes cardiotoxicity and waste of money. Therefore, accurate determination of HER-2 status in breast cancer patients is an important part of routine practice in pathological reporting. Cases with weak positive staining (2+) by HER-2 IHC represent a subgroup of patients that requires additional assessment with FISH.
A variety of IHC antibodies and other methods have been developed to determine HER-2 status in breast cancer patients. Ciftlik et al. (21) designed a glass/silicon micro-machined structure for applying microfluidic tissue processing protocols, thus allowing rapid IHC processing of breast carcinomas and correct determination of HER-2 status. The concordance rate between microfluidic processor results and subsequent in situ hybridization (ISH) of the same samples was 100%, although the number of cases included in this study was relatively small (score IHC 2+, n=27). SP3, a rabbit monoclonal antibody, was proven to have a high level of agreement with ISH methods (22). The concordance rates reported by D’Alfonso (23) from 100 breast cancer patients between SP3 and FISH in needle core biopsy and excisional biopsy specimens were 96% (95% CI, 91.9-99.7%) and 97% (95% CI, 90.3-99.3%), respectively. Despite the steps that have been made to standardize the process of IHC assessment, intra- and inter-observer variability in scoring is not uncommon (24). Computer-assisted analysis on HER-2 IHC slides may be an effective supplement to conventional IHC analysis (25); however, this method requires special materials and could not be widely implemented for use within a short time.
Although numerous previous studies have reported that HER-2 overexpression (IHC 3+) or HER-2 amplification is associated with high tumor cell grade, absence of ER or PR expression, DNA aneuploidy, and high Ki67 (26-29), published evidence on the correlation between relevant prognostic factors and FISH-determined HER-2 status in HER-2 IHC 2+ cases is still lacking. A method with high discriminatory power will help clinical physicians obtain results faster without the performance of FISH. To date, only three publications have studied this relationship. Lee (30) recently characterized a relatively large series of 1735 invasive breast cancer tissues, among which 419 (24%) were scored HER-2 2+ by IHC. Additionally, 14% (57/413) were HER-2 amplified according to FDA criteria (ratio of HER-2 to chromosome 17≥2.0). HER-2 amplification was related to the percentage of complete membrane staining. Chibon (31) selected 108 breast cancers with HER-2 IHC score of 2+ to predict HER-2 gene status. FISH amplification rate was determined to be 33%. Tumor grade and percentage of membrane staining were indicators of HER-2 status. A study by Dieci et al. (32) analyzed 480 HER-2 2+ breast cancer samples, resulting in high tumor grade and high Ki67 being significantly associated with HER-2 FISH amplification. However, the ER and PR statuses were not determined in all cases. HR positivity is related with better prognosis in breast cancer patients. Furthermore, although the association between pathological variables (tumor grade and Ki67) and HER-2 status has been well established, the power of these studies has been relatively low. To ensure that all women with HER-2 amplified cancers receive adequate treatment, a powerful method for assessing HER-2 amplification is imperative. In our study, we integrated clinical and pathological factors from 182 invasive breast cancer cases with IHC score of 2+ to develop a risk score that better predicts the occurrence of HER-2 amplification. All samples were routinely submitted for FISH analysis to determine the HER-2 gene status. We found that 34.6% (63/182) of all cases were HER-2 amplified. A positive correlation was found between the HR, P53, and Ki67 and HER-2 status. The risk score, derived by the sum of HR, P53, and Ki67, was a highly significant predictor of HER-2 status (χ2=30.41, P<0.001). Overall, compared with previous studies, this study examined cases that were all from surgical specimens, and incorporated multiple clinicopathological parameters for the development of a powerful predictive model for HER-2 status. The additional variables allow for higher accuracy for validation of HER-2 status.
Some limitations were observed in this study. First, our analysis focused on invasive breast cancer, thereby limiting our analysis from other histological classifications; second, this study was based on patients from one center, and results may not apply to other medical settings. Before clinical use, the evaluation of ER, PR, Ki67 should be standardized; third, any predictive model incorporates a certain degree of uncertainty, so predicting the status of an individual patient remains imperfect. More studies that address these issues are needed for confirmation. Despite the statistical accuracy for the prediction of HER-2 amplification in invasive breast cancer, FISH analysis remains the gold standard for determining HER-2 status.
Accurately evaluating the breast cancer HER-2/neu genotype has become an important task as emerging date showing that the benefit of using Herceptin in the treatment for HER-2 positive patients. Subgroup of breast cancer patients achieves a pCR after the neoadjuvant chemotherapy. There is no residual tumor cell in the surgical biopsy for examination. Tissue accessibility prohibits patients from obtaining HER-2 status. Preoperation needle core biopsy tissue becomes the only available material in this group of patients. In such cases, our risk score can be used to prioritise the treatment of Herceptin. In a recently meta-analysis (33), HER-2 IHC 0/1+ and 3+ cannot be absolutely considered as negative and positive. The discordance rates are 4% and 9% in 0/1+ and 3+ HER-2 IHC score, respectively. In such instances, this IHC risk score would help physician to select those patients who will benefit from the target therapy.
Based on the results of our study, we present a novel IHC risk score that will help determine HER-2 status accurately. In the future, we hope to validate this model by analyzing a larger series of invasive breast cancer tissues.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ali AM, Provenzano E, Bartlett JM, et al. Prognosis of early breast cancer by immunohistochemistry defined intrinsic sub-types in patients treated with adjuvant chemotherapy in the NEAT/BR9601 trial. Int J Cancer 2013;133:1470-8. [PubMed]
- Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747-52. [PubMed]
- López-Guerrero JA, Llombart-Cussac A, Noguera R, et al. HER2 amplification in recurrent breast cancer following breast-conserving therapy correlates with distant metastasis and poor survival. Int J Cancer 2006;118:1743-9. [PubMed]
- Ithimakin S, Day KC, Malik F, et al. HER2 drives luminal breast cancer stem cells in the absence of HER2 amplification: implications for efficacy of adjuvant trastuzumab. Cancer Res 2013;73:1635-46. [PubMed]
- Witton CJ, Reeves JR, Going JJ, et al. Expression of the HER1-4 family of receptor tyrosine kinases in breast cancer. J Pathol 2003;200:290-7. [PubMed]
- Press MF, Sauter G, Bernstein L, et al. Diagnostic evaluation of HER-2 as a molecular target: an assessment of accuracy and reproducibility of laboratory testing in large, prospective, randomized clinical trials. Clin Cancer Res 2005;11:6598-607. [PubMed]
- Persons DL, Borelli KA, Hsu PH. Quantitation of HER-2/neu and c-myc gene amplification in breast carcinoma using fluorescence in situ hybridization. Mod Pathol 1997;10:720-7. [PubMed]
- Tandon AK, Clark GM, Chamness GC, et al. HER-2/neu oncogene protein and prognosis in breast cancer. J Clin Oncol 1989;7:1120-8. [PubMed]
- Holmes P, Lloyd J, Chervoneva I, et al. Prognostic markers and long-term outcomes in ductal carcinoma in situ of the breast treated with excision alone. Cancer 2011;117:3650-7. [PubMed]
- Di Leo A, Desmedt C, Bartlett JM, et al. HER2 and TOP2A as predictive markers for anthracycline-containing chemotherapy regimens as adjuvant treatment of breast cancer: a meta-analysis of individual patient data. Lancet Oncol 2011;12:1134-42. [PubMed]
- Reis-Filho JS, Pusztai L. Gene expression profiling in breast cancer: classification, prognostication, and prediction. Lancet 2011;378:1812-23. [PubMed]
- Nielsen DL, Kümler I, Palshof JA, et al. Efficacy of HER2-targeted therapy in metastatic breast cancer. Monoclonal antibodies and tyrosine kinase inhibitors. Breast 2013;22:1-12. [PubMed]
- Davoli A, Hocevar BA, Brown TL. Progression and treatment of HER2-positive breast cancer. Cancer Chemother Pharmacol 2010;65:611-23. [PubMed]
- Jelovac D, Emens LA. HER2-directed therapy for metastatic breast cancer. Oncology (Williston Park) 2013;27:166-75. [PubMed]
- Esteva FJ, Franco SX, Hagan MK, et al. An open-label safety study of lapatinib plus trastuzumab plus paclitaxel in first-line HER2-positive metastatic breast cancer. Oncologist 2013;18:661-6. [PubMed]
- Mendoza G, Portillo A, Olmos-Soto J. Accurate breast cancer diagnosis through real-time PCR her-2 gene quantification using immunohistochemically-identified biopsies. Oncol Lett 2013;5:295-8. [PubMed]
- Going JJ. Observer prediction of HER2 amplification in HercepTest 2+ breast cancers as a potential audit instrument. Histopathology 2011;59:333-5. [PubMed]
- Lee AH, Key HP, Bell JA, et al. Breast carcinomas with borderline (2+) HER2 immunohistochemistry: percentage of cells with complete membrane staining for HER2 and the frequency of HER2 amplification. J Clin Pathol 2011;64:490-2. [PubMed]
- Chibon F, de Mascarel I, Sierankowski G, et al. Prediction of HER2 gene status in Her2 2+ invasive breast cancer: a study of 108 cases comparing ASCO/CAP and FDA recommendations. Mod Pathol 2009;22:403-9. [PubMed]
- Rakha EA, Martin S, Lee AH, et al. The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer 2012;118:3670-80. [PubMed]
- Ciftlik AT, Lehr HA, Gijs MA. Microfluidic processor allows rapid HER2 immunohistochemistry of breast carcinomas and significantly reduces ambiguous (2+) read-outs. Proc Natl Acad Sci U S A 2013;110:5363-8. [PubMed]
- Nunes CB, Rocha RM, Reis-Filho JS, et al. Comparative analysis of six different antibodies against Her2 including the novel rabbit monoclonal antibody (SP3) and chromogenic in situ hybridisation in breast carcinomas. J Clin Pathol 2008;61:934-8. [PubMed]
- D’Alfonso TM, Liu YF, Chen Z, et al. SP3, a reliable alternative to HercepTest in determining HER-2/neu status in breast cancer patients. J Clin Pathol 2013;66:409-14. [PubMed]
- Press MF, Sauter G, Bernstein L, et al. Diagnostic evaluation of HER-2 as a molecular target: an assessment of accuracy and reproducibility of laboratory testing in large, prospective, randomized clinical trials. Clin Cancer Res 2005;11:6598-607. [PubMed]
- Hall BH, Ianosi-Irimie M, Javidian P, et al. Computer-assisted assessment of the human epidermal growth factor receptor 2 immunohistochemical assay in imaged histologic sections using a membrane isolation algorithm and quantitative analysis of positive controls. BMC Med Imaging 2008;8:11. [PubMed]
- Hussein MR, Abd-Elwahed SR, Abdulwahed AR. Alterations of estrogen receptors, progesterone receptors and c-erbB2 oncogene protein expression in ductal carcinomas of the breast. Cell Biol Int 2008;32:698-707. [PubMed]
- Lal P, Tan LK, Chen B. Correlation of HER-2 status with estrogen and progesterone receptors and histologic features in 3,655 invasive breast carcinomas. Am J Clin Pathol 2005;123:541-6. [PubMed]
- Liu C, Zhang H, Shuang C, et al. Alterations of ER, PR, HER-2/neu, and P53 protein expression in ductal breast carcinomas and clinical implications. Med Oncol 2010;27:747-52. [PubMed]
- Hanley K, Wang J, Bourne P, et al. Lack of expression of androgen receptor may play a critical role in transformation from in situ to invasive basal subtype of high-grade ductal carcinoma of the breast. Hum Pathol 2008;39:386-92. [PubMed]
- Lee AH, Key HP, Bell JA, et al. Breast carcinomas with borderline (2+) HER2 immunohistochemistry: percentage of cells with complete membrane staining for HER2 and the frequency of HER2 amplification. J Clin Pathol 2011;64:490-2. [PubMed]
- Chibon F, de Mascarel I, Sierankowski G, et al. Prediction of HER2 gene status in Her2 2+ invasive breast cancer: a study of 108 cases comparing ASCO/CAP and FDA recommendations. Mod Pathol 2009;22:403-9. [PubMed]
- Dieci MV, Barbieri E, Bettelli S, et al. Predictors of human epidermal growth factor receptor 2 fluorescence in-situ hybridisation amplification in immunohistochemistry score 2+ infiltrating breast cancer: a single institution analysis. J Clin Pathol 2012;65:503-6. [PubMed]
- Bahreini F, Soltanian AR, Mehdipour P. A meta-analysis on concordance between immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) to detect HER2 gene overexpression in breast cancer. Breast Cancer 2014. [Epub ahead of print]. [PubMed]


