
Prognosis of upstaged N1 and N2 disease after curative resection in patients with clinical N0 non-small cell lung cancer
Introduction
The standard treatment of stage I non-small cell lung cancer (NSCLC) is surgical resection (1). Surgery is the first choice of treatment followed by adjuvant chemotherapy (2,3) in N1 NSCLC. However, for patients with clinical N2 NSCLS, the National Comprehensive Cancer Network guideline for NSCLC (Version 2. 2019) recommends neoadjuvant chemotherapy. Therefore, accurate clinical staging is considered important in determining the treatment plan. For lung cancer staging, imaging studies such as chest computed tomography (CT) and positron emission tomography (PET) are mainly used. Preoperative invasive lymph node staging procedures, including endobronchial ultrasonography (EBUS), transbronchial needle aspiration (TBNA), esophageal ultrasonography (EUS), fine-needle aspiration (FNA), or mediastinoscopy, would also be used for precise lymph node staging. However, invasive lymph node staging is not always necessary for patients with no detectable lymph node metastases on imaging studies, and it is questionable whether aggressive invasive lymph node staging affects the prognosis of patients with clinical stage I disease on imaging.
If the patient considering surgery was diagnosed with clinical T1–2N0 NSCLC by imaging studies, would anatomical resection and mediastinal lymph node dissection be sufficient without further preoperative evaluation of the lymph nodes? In other words, if nodal upstaging is found after surgery, would the patient’s prognosis be improved by discovery of the lymph node metastasis before surgery?
The purpose of this study was to evaluate the prognosis of upstaged N1 and N2 disease from clinical N0 NSCLC. We compared the prognosis of upstaged N1 and N2 NSCLC with that of pre-operatively confirmed N1 and N2 NSCLC to determine whether the prognosis was affected by surgery alone without further preoperative invasive lymph node examination. Through this approach, we investigated whether surgery, without preoperative pathologic lymph node staging, is acceptable for clinical T1-2 N0 NSCLC by imaging.
Methods
Patients
From May 2005 to December 2015, 1,352 consecutive patients at Seoul St. Mary’s Hospital in Korea were diagnosed with NSCLC and underwent therapeutic surgical resection. Of those patients, 676 were diagnosed with clinical N0 (T1–2, tumor size ≤5 cm) NSCLC and underwent curative resection. Resection was complete in all cases, and no preoperative chemo- or radiotherapy was administered. Patients with synchronous lung cancer or multiple nodules were excluded. Following resection, 46 of the clinical N0 tumors were upstaged to N1 and 24 were upstaged to N2. Patients with tumors that were upstaged from clinical N0 to N1 were defined as the upstaged N1 group and those with clinical N0 tumors that were upstaged to N2 were defined as the upstaged N2 group. Cases of nodal upstaging from N1 to N2 were not included in the upstaged N2 group. For comparison of prognosis between patients with upstaged tumors and others in the same stage, patients with preoperatively proven T1-2 N1 (non-upstaged N1 group, n=31) and N2 (non-upstaged N2 group, n=55) tumors (single lesion, tumor size ≤5 cm) were included in this study. Only patients with T1 to T2 (tumor size ≤5 cm and single lesion) tumors were included because stage T3N2 (stage IIIB) disease is not considered operable, and patients in the non-upstaged N1 or N2 group who had incomplete resection or remnant lymph node metastasis were also excluded. Anatomical resections (lobectomy, bilobectomy, and pneumonectomy) were performed in all patients with mediastinal lymph node dissection and en bloc resection of lymph nodes and adjacent fat tissue at more than three mediastinal lymph node stations. Resection was complete in all the study patients; no patients with incomplete resections were included. A total of 156 consecutive cases were reviewed retrospectively for clinicopathological characteristics and prognosis. The study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital, Catholic University of Korea (KC18RESI0024).
Preoperative staging and lymph node evaluation
Preoperative lymph node staging was performed using contrast-enhanced chest CT and F-18-fluorodeoxyglucose (FDG) PET/CT scanning. Diagnostic findings for stage N1 and N2 lymph node metastases included short-axis diameters exceeding 10 mm on CT scan and nodal FDG uptake surpassing that of surrounding mediastinal structures on PET/CT. However, a lymph node with high FDG uptake was considered benign if the lymph node contained benign calcifications or if unenhanced CT images showed high attenuation with a distinct margin (4,5). A generally symmetric and equivocal FDG uptake in the mediastinal lymph nodes on PET/CT was interpreted as a reactive inflammatory change in the lymph node. If complete resection was considered feasible, patients with clinical N0 tumors on chest CT and PET/CT scan went to surgery without invasive preoperative lymph node staging. Consequently, we did not perform invasive preoperative lymph node staging in any patients with clinical N0 NSCLC.
Histologic evaluation
All clinical specimens were examined by pathologists and their observations were recorded. Each patient report was reviewed for tumor size, location, lymph node status, pleural invasion, and lymphovascular invasion (LVI). Central lung lesions were defined as tumor location limited to the trachea, bronchi, or segmental bronchi, and peripheral lesions were defined as tumor location limited more to the periphery than to the subsegmental bronchi (6). TNM staging was based on the 8th edition TNM classification (7).
Statistical analysis
We compared the clinicopathological characteristics of patients in each upstaged group with those of patients in each non-upstaged N1/N2 group. Student’s t-test or Wilcoxon rank-sum test was used for continuous variables and Chi-square or Fisher’s exact test was applied for categorical variables. The Kaplan-Meier method was used to calculate the interval from surgical resection until the final follow-up visit, using confirmed recurrences and cancer-related deaths for recurrence-free survival (RFS) and disease-specific survival (DSS). RFS and DSS in the upstaged groups vs. the non-upstaged N1/N2 groups were compared by log-rank test. Cox proportional hazards model was used in a multivariate analysis to determine the risk of cancer-related death for all patients. P values below 0.05 were considered statistically significant. Statistical calculations were performed using SPSS version 24.0 (IBM Corporation, Armonk, NY).
Results
Among all 676 patients with clinical N0 tumors, 70 (10.4%) had nodal upstaging after curative resection, 46 to N1 and 24 to N2. Comparisons of the clinicopathological characteristics between upstaged and non-upstaged N1/N2 groups are shown in Tables 1,2. The comparisons of RFS and DSS between the groups are shown in Figures 1,2. The median follow-up time for all patients was 1,379 days (range: 12–4,579 days).
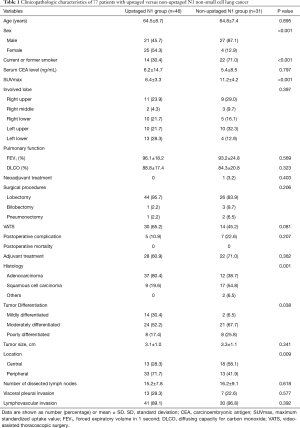
Full table
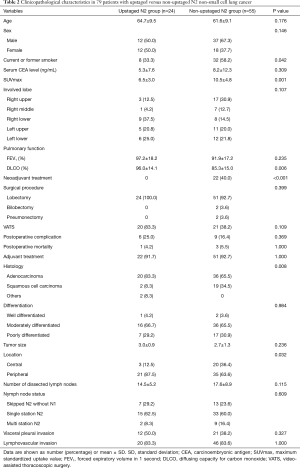
Full table
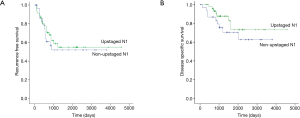
Comparison of prognosis between upstaged and non-upstaged N1 groups
Five-year RFS (54.9% vs. 51.9%; P=0.648) and DSS (73.3% vs. 70.5%, P=0.247) were not significantly different between patients with upstaged (n=46) and non-upstaged (n=31) N1 tumors (Figure 1), and recurrence sites were also similar (Table 3). However, there were differences between the groups in clinicopathological characteristics, including gender, smoking status, maximum standardized uptake value (SUVmax) on PET/CT, video-assisted thoracoscopic surgery (VATS), histologic type, tumor differentiation, and tumor location (Table 1). Therefore, it was difficult to conclude that nodal upstaging had no effect on prognosis in N1 NSCLC. Even with similar survival between the upstaged N1 group and the non-upstaged N1 group, the clinicopathological factors between the two groups are not the same. Univariate and multivariate analyses using a Cox proportional hazard model were conducted to identify the prognostic factors. In univariate analysis, there were no significant prognostic factors in N1 lung cancer. The clinicopathological factors that differed between the upstaged N1 group and the non-upstaged N1 group were included in a multivariate analysis with a new variable, nodal upstaging (Table 4), to confirm the effect of nodal upstaging on prognosis in N1 lung cancer. The multivariate analysis was conducted to identify risk factors for cancer-related death, and no significant risk factor for cancer-related death was identified. Thus, based on our comparison of survival rates and the multivariate analysis, it appears that nodal upstaging is not a significant prognostic factor in N1 NSCLC [hazard ratio (HR) =0.385; 95% confidence interval (CI), 0.080–1.860; P=0.235].

Full table
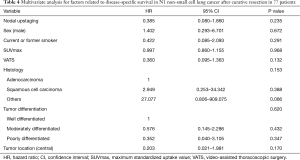
Full table
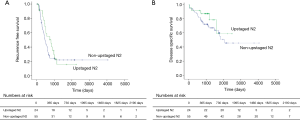
Comparison of prognosis between upstaged and non-upstaged N2 groups
We also compared survival between upstaged (n=24) and non-upstaged (n=55) N2 groups. Five-year RFS (15.6% vs. 22.0%, P=0.356) and 5-year DSS (58.9% vs. 50.7%, P=0.283) were similar (Figure 2), as were sites of recurrence (Table 5). However, certain clinicopathological characteristics differed between the N2 groups, including smoking status, SUVmax, diffusing capacity for carbon monoxide (DLCO), histologic type, and tumor location (Table 2). Therefore, even without a significant difference in survival between the upstaged and non-upstaged N2 groups, it was difficult to conclude that nodal upstaging had no effect on prognosis in N2 NSCLC because the clinicopathological factors between the two groups are not the same. Univariate and multivariate analyses were conducted using a Cox proportional hazard model to identify prognostic factors. In univariate analysis, there were no significant prognostic factors in N2 lung cancer. The clinicopathological factors that differed between the two groups were included in multivariate analysis, along with the new variable, nodal upstaging, to confirm the effect of nodal upstaging on prognosis in N2 NSCLC (Table 6). The multivariate analysis revealed no statistically significant risk factor for cancer-related death. Thus, based on our comparison of survival rates and multivariate analysis, nodal upstaging was not a significant prognostic factor in N2 NSCLC (HR =0.677; 95% CI, 0.242–1.895; P=0.458).

Full table
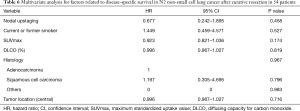
Full table
Discussion
Surgery is the primary treatment option in early-stage lung cancer. However, surgery alone is not considered an effective treatment for advanced lung cancer (8). Therefore, a multidisciplinary team approach for lung cancer is crucial, with optimum treatment methods to be determined according to the clinical stage. As imaging techniques such as chest CT and PET/CT have become more advanced, the reliability of cancer staging through imaging information is improving. Our hospital is a tertiary hospital in Korea with the latest facilities, and we trust the imaging techniques that we use in lung cancer staging. Therefore, in patients with clinical N0 NSCLC on imaging, preoperative invasive lymph node staging is omitted and surgical resection is performed immediately. When postoperative nodal upstaging is occasionally found, adjuvant treatment is offered after surgery.
The European Society of Thoracic Surgeons suggests that direct surgery without invasive mediastinal lymph node staging can be performed if there are no suspect lymph nodes on CT or PET scan, the tumor size is 3 cm or smaller, and the and tumor is located in the outer third of the lung (9). In our institution, however, surgery was performed without preoperative invasive lymph node staging in all cases of clinical N0 NSCLC diagnosed by chest CT and PET/CT imaging studies alone, because we believe that clinical N0 lymph nodes can be removed by curative anatomical resection and mediastinal lymph node dissection. A lymph node that is clinically N0 on chest CT and PET/CT scan is small and has little surrounding invasion. Therefore, it is easily removed by surgery even if it contains occult metastases.
We found that there were no significant differences in 5-year survival rates between upstaged and non-upstaged N1 and N2 groups. Notably, 40% of patients in the non-upstaged N2 group had received neoadjuvant chemotherapy, but recurrence and cancer-related death did not differ between upstaged N2 without neoadjuvant chemotherapy and non-upstaged N2. Although there were some clinicopathological differences between the upstaged and non-upstaged N1/N2 groups, nodal upstaging did not affect survival in multivariate analysis. Therefore, the use of neoadjuvant treatment for occult N2 disease detected by invasive preoperative nodal staging in patients with clinical N0 NSCLC may not help to improve survival rates.
Of course, this study was not intended to examine the efficacy of neoadjuvant chemotherapy. To determine whether neoadjuvant chemotherapy is effective in an upstaged group, we must compare the survival of patients with or without neoadjuvant chemotherapy in the same upstaged group. Because no patient with clinical N0 NSCLC received neoadjuvant chemotherapy, we could not make such a comparison. In the future, comparative analysis may be possible through prospective randomized controlled trials. However, since nodal upstaging from clinical N0 is infrequent, such comparative studies are very limited in practice.
The incidence of nodal upstaging after surgery in clinical stage I NSCLC varies (5,10-13). Heineman et al. reported lymph node upstaging in 14.9% of patients with clinical stage I NSCLC after anatomical resection (14). Kirmani et al. reported lymph node upstaging in 13.0% of patients with T1N0M0 NSCLC and 25.7% of patients with T2N0M0 NSCLC (15). Samson et al. used the United States National Cancer Database to show an incidence of lymph node upstaging of 12.6% in clinical stage I NSCLC (16), and Ye et al. found that the rate of lymph node upstaging in clinical stage T1N0M0 lung adenocarcinoma was 10.6% (17). The present study included clinical N0 NSCLC (tumor size ≤5 cm) diagnosed by chest CT and PET/CT scan, and the incidence of lymph node upstaging was 10.4%. Thus, our results are comparable to those of other studies, and we believe our preoperative clinical staging was conducted appropriately.
Risk factors for nodal upstaging despite a finding of clinical N0 by preoperative imaging have also been analyzed (5,17-21). The European Society of Thoracic Surgeons guideline recommends invasive preoperative lymph node staging when the probability of mediastinal lymph node metastasis seems greater, as in central location, larger than 3 cm, and suspected N1 metastasis (9). Other studies have shown a greater likelihood of nodal upstaging when the tumor has high SUVmax or a specific histologic type. In such cases, preoperative invasive mediastinal lymph node staging may be helpful. However, our results suggest that in clinical N0 NSCLC that is considered completely resectable on imaging studies, invasive preoperative nodal staging may not be required. This finding should not preclude preoperative invasive nodal staging. Apart from this study, it is not meaningless to predict nodal upstaging in clinical N0 NSCLC. For example, if nodal upstaging can be predicted in advance, it is possible to avoid limited treatments such as sublobar resection or stereotactic ablative radiotherapy (22).
This study had some limitations. First, it was a retrospective review. Second, we obtained data from a single institution and there was an insufficient sample size to generalize our results. However, this study examined data from surgical patients under a relatively standardized protocol at our single center, a tertiary hospital in Korea. Furthermore, a more detailed analysis was possible due to the comprehensive data available in the electronic medical records. Third, the comparison of survival between the two groups was not sufficient because of the heterogeneity of clinicopathological characteristics between the groups. Nevertheless, this study was meaningful in confirming the survival rate of upstaged N1/N2 lung cancer, and we believe that our findings can be used as a basis for future investigations. A larger scale study should be performed to validate our results.
In conclusion, postoperative nodal upstaging from clinical N0 NSCLC was not a significant prognostic factor in the same stage group. Recurrence-free survival and disease-specific survival were not different between patients with upstaged and non-upstaged N1 and N2 NSCLC. Therefore, surgical treatment for clinical T1–2N0 lung cancer based only on imaging studies without preoperative pathologic lymph node staging can be a treatment option. A larger scale study with a more homogenous cohort should be performed to validate our results.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Pisters KM, Evans WK, Azzoli CG, et al. Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIA resectable non small-cell lung cancer guideline. J Clin Oncol 2007;25:5506-18. [Crossref] [PubMed]
- Berry MF, Coleman BK, Curtis LH, et al. Benefit of adjuvant chemotherapy after resection of stage II (T1-2N1M0) non-small cell lung cancer in elderly patients. Ann Surg Oncol 2015;22:642-8. [Crossref] [PubMed]
- Lu P, Sun Y, Sun Y, et al. The role of (18)F-FDG PET/CT for evaluation of metastatic mediastinal lymph nodes in patients with lung squamous-cell carcinoma or adenocarcinoma. Lung Cancer 2014;85:53-8. [Crossref] [PubMed]
- Moon Y, Kim KS, Lee KY, et al. Clinicopathologic Factors Associated With Occult Lymph Node Metastasis in Patients With Clinically Diagnosed N0 Lung Adenocarcinoma. Ann Thorac Surg 2016;101:1928-35. [Crossref] [PubMed]
- Moon Y, Lee KY, Sung SW, et al. Differing histopathology and prognosis in pulmonary adenocarcinoma at central and peripheral locations. J Thorac Dis 2016;8:169-77. [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Douillard JY, Tribodet H, Aubert D, et al. Adjuvant cisplatin and vinorelbine for completely resected non-small cell lung cancer: subgroup analysis of the Lung Adjuvant Cisplatin Evaluation. J Thorac Oncol 2010;5:220-8. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Moon Y, Sung SW, Namkoong M, et al. The effectiveness of mediastinal lymph node evaluation in a patient with ground glass opacity tumor. J Thorac Dis 2016;8:2617-25. [Crossref] [PubMed]
- Suh JH, Park JK, Moon Y. Prognostic prediction of clinical stage IA lung cancer presenting as a pure solid nodule. J Thorac Dis 2018;10:3005-15. [Crossref] [PubMed]
- Moon Y, Park JK, Lee KY, et al. Consolidation/Tumor Ratio on Chest Computed Tomography as Predictor of Postoperative Nodal Upstaging in Clinical T1N0 Lung Cancer. World J Surg 2018;42:2872-8. [Crossref] [PubMed]
- Moon Y, Lee KY, Kim KS, et al. Clinicopathologic correlates of postoperative N1 or N2 nodal upstaging in non-small cell lung cancer. J Thorac Dis 2016;8:79-85. [PubMed]
- Heineman DJ, Ten Berge MG, Daniels JM, et al. Clinical Staging of Stage I Non-Small Cell Lung Cancer in the Netherlands-Need for Improvement in an Era With Expanding Nonsurgical Treatment Options: Data From the Dutch Lung Surgery Audit. Ann Thorac Surg 2016;102:1615-21. [Crossref] [PubMed]
- Kirmani BH, Rintoul RC, Win T, et al. Stage migration: results of lymph node dissection in the era of modern imaging and invasive staging for lung cancer. Eur J Cardiothorac Surg 2013;43:104-9; discussion 109-10. [Crossref] [PubMed]
- Samson P, Crabtree T, Broderick S, et al. Quality Measures in Clinical Stage I Non-Small Cell Lung Cancer: Improved Performance Is Associated With Improved Survival. Ann Thorac Surg 2017;103:303-11. [Crossref] [PubMed]
- Ye B, Cheng M, Li W, et al. Predictive factors for lymph node metastasis in clinical stage IA lung adenocarcinoma. Ann Thorac Surg 2014;98:217-23. [Crossref] [PubMed]
- Ghaly G, Rahouma M, Kamel MK, et al. Clinical Predictors of Nodal Metastases in Peripherally Clinical T1a N0 Non-Small Cell Lung Cancer. Ann Thorac Surg 2017;104:1153-8. [Crossref] [PubMed]
- Miyasaka Y, Suzuki K, Takamochi K, et al. The maximum standardized uptake value of fluorodeoxyglucose positron emission tomography of the primary tumour is a good predictor of pathological nodal involvement in clinical N0 non-small-cell lung cancer. Eur J Cardiothorac Surg 2013;44:83-7. [Crossref] [PubMed]
- Li M, Wu N, Zheng R, et al. Primary tumor PET/CT [(1)(8)F]FDG uptake is an independent predictive factor for regional lymph node metastasis in patients with non-small cell lung cancer. Cancer Imaging 2013;12:566-72. [Crossref] [PubMed]
- Yamazaki K, Yoshino I, Yohena T, et al. Clinically predictive factors of pathologic upstaging in patients with peripherally located clinical stage IA non-small cell lung cancer. Lung Cancer 2007;55:365-9. [Crossref] [PubMed]
- Moon Y, Lee KY, Park JK. Prognosis After Sublobar Resection of Small-sized Non-small Cell Lung Cancer with Visceral Pleural or Lymphovascular Invasion. World J Surg 2017;41:2769-77. [Crossref] [PubMed]


