The effect of primary graft dysfunction after lung transplantation on parenchymal remodeling detected by quantitative computed tomography
Introduction
Primary graft dysfunction (PGD) is a syndrome related to allograft ischemia-reperfusion injury that occurs within the first 72 hours after lung transplantation. In the early days of lung transplantation, the incidence of PGD is difficult to assess accurately, with a reported range varying from 15% to 57%, partly as a result of differing definitions (1,2). The diagnosis is based on the presence of diffuse alveolar infiltrates on chest radiography without other identifiable causes and graduated on PaO2 fraction of inspired oxygen (FiO2) value (3,4). Notably, PGD is a risk factor for early and 1-year mortality; in addition, PGD is related to the development of bronchiolitis obliterans syndrome (5). Conversely, its role in association with functional outcomes is less clear (3,6) and the evolution of tissue damaged from the ischemia-reperfusion injury has only sporadically been studied (7). In this scenario, functional analysis of multi-volumetric computed tomography (CT) represents an attractive tool that offers the possibility to study and understand the ‘parenchymal outcome’ after lung transplantation, opening the door to patient-tailored management (8).
The overall purpose of this pilot study was to determine whether development of PGD in the first 72 hours after lung transplantation influences parenchymal remodeling quantitatively assessed by CT at 3- and 12-month follow-up.
Methods
Study design
We designed a prospective, observational, single blind pilot study, based on dedicated institutional database. The study was performed in compliance with the principles of the declaration of Helsinki and received the approval of the local ethics committee (749_2016bis). All participants gave written informed consent before they were included in the study.
Patient population
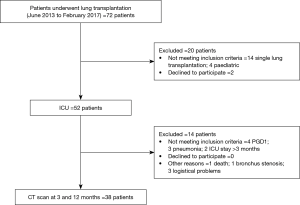
We prospectively enrolled all patients who underwent bilateral lung transplantation at Fondazione IRCCS Ca’ Granda-Ospedale Maggiore Policlinico of Milan between June 2013 and February 2017. Exclusion criteria were age <18 years and consent denied.
PGD diagnosis and grading
Patients were evaluated at 24, 48 and 72 hours after the end of surgery to establish PGD occurrence and grading. Two Authors (M Nosotti, A Palleschi) examined independently the chest X-ray. According to the standard ISHLT criteria, given the multi-factorial and likely additive nature of PGD risk factors, if parenchymal infiltrates were present, every other possibility was investigated and excluded (4). If any discrepancy occurred between the researchers, they proceeded to a collegial review of clinical and radiological parameters. PGD was graded on the basis of PaO2/FiO2 ratio and recorded on the database. Patients were classified in two groups according the worst PGD within the first 72 hours. Patients without evidence of PGD constituted the PGD0 Group; patients with grade 2 and/or 3 graft dysfunction within first 72 hours after lung transplantation composed the PGD Group.
Surveillance protocol
Besides fifteen-day medical examination, our standard surveillance protocol included pulmonary function tests (PFTs), multi-volumetric CT scans, and bronchoscopy with trans-bronchial biopsy at 3, 6 and 12 months. All data of interest were recorded in the dedicated database.
Specifically, PFT consists in a spirometry (Spirolab, Medical International Research, Italy) performed on the same day of the CT scan. This test included the measure of forced-expiratory volume in 1 second (FEV1) and forced vital capacity (FVC); the values were expressed also as percentage of predicted (FEV1%, FVC %).
Patients underwent low dose, non-contrast CT scan with BrightSpeed™ Elite SD (GE Healthcare, CT, USA). CT images were acquired during breath holding at full end-inspiration (INSP) and full end-expiration (EXP) with the following parameters: tube voltage, 100 kVp, reference tube current, 245 mAs, pitch factor, 1.75, image matrix, 512×512, slice thickness 2.5 mm, with a smooth filter (B30). The DLP (Dose Length Product) was in the range between 110 and 328 mGy/cm, and the CTDIVol (Computed Tomography Dose Index Vol) was in the range between 1.8 and 4.9 mGy which is a low radiation dose compared to the doses used for routine chest scanning in many institutions (9).
Quantitative image analysis
For the aim of the study, we considered the surveillance CT scans at 3 and 12 months (±10 days) after transplantation. Image analysis was performed, in terms of specific gas volume (SVg) for the entire lung. SVg is defined as the difference between specific volume of tissue and gas and the specific volume of tissue, expressed in mL (gas)/g (tissue) (10-13). For the calculation of SVg, voxels belonging to airways, blood vessels, and other structures of the lung were excluded and only those belonging to the parenchyma, i.e., with Hounsfield Unit values <−600 HU, were considered. Changes of SVg (ΔSVg = SVgINSP − SVgEXP, expressed as mL·g−1, where SVgINSP and SVgEXP are specific gas volume at EXP and INSP, respectively) normalized on SVgEXP were calculated as ΔSVg/SVgEXP. Additional measures included CT total lung volumes (VEXP and VINSP) at each time point, as described elsewhere (9,10,12).
CT functional mask
In order to identify ventilation defects on a pixel-by-pixel basis, individual maps of CT density change between the two lung volumes (ΔHU = HUEXP − HUINSP) were obtained on five different lung levels, from the aortic arch to the top of the diaphragm. Firstly, INSP and EXP images were semi-automatically segmented to separate lung parenchyma from the surrounding tissues. Successively, expiratory images were automatically deformed to the corresponding inspiratory images by using the Lucas-Kanade optical-flow algorithm (14,15) and then subtracted pixel-by-pixel, providing maps of ΔHU (10). Each pixel was then classified as belonging to four different possible functional settings: (I) low ventilated (green), (II) consolidation (blue), (III) air trapping (red) and (IV) healthy regions (black).
The change in density was calculated for each voxel of the ΔHU map and the classification was determined by imposing four thresholds: (I) the threshold for healthy regions (H, in black) was determined by considering a variation greater or equal to 100 (16); (II) pixels with values less than −950 HU on inspiration (HUINSP <−950 HU) and less than −856 HU on expiration images (HUEXP <−856) in conjunction with ΔHU <100 denoted air trapping (AT, in red) (8); (III) ΔHU <100, in conjunction with either HUEXP >−856 or HUINSP >−950 HU designated low ventilated regions (LV, in green) and (IV) ΔHU <100, in conjunction with HUEXP and HUINSP >−400 HU denoted the areas of abnormally high attenuation (C, in blue).
The percentages of LV, C, AT and H regions with respect to total area in the analyzed slices, denoted as %LV, %C, %AT and %H, respectively were calculated across the five ΔHU maps.
All algorithms for image processing and quantitative analysis were implemented by custom software developed in MATLAB (The MathWorksInc, Natick, MA).
Statistical analysis
Statistical analysis was performed using SigmaStat version 12.5 (Systat Software, San Jose, CA, USA). Bivariate analyses were conducted using Mann Whitney’s U-test for continuous variables and chi-square or Fisher’s exact tests for categorical variables. Paired t-test was used to assess differences between time points and groups. A one-tailed paired t-test was performed to analyze the extent of change in ΔSVg/SVgEXP and %LV between groups. Regression analyses were performed to correlate the relative change in VEXP/VINSP and ΔSVg/SVgEXP and %LV. A P<0.05 was considered statistically significant.
Results
The study population is shown in Figure 1. Namely, 38 subjects were included in this study; there were 13 males and 25 females, with a median age of 38 years. The characteristics of the patients are summarized in Table 1.

Full table
Functional outcomes
Three months after lung transplantation, PFTs showed that patients with or without PGD had statistically different reached values (FEV1% 64±13 vs. 79±19, P=0.014; FVC% 65±11 vs. 77±16, P=0.032); conversely, comparable results were obtained at 12 months (FEV1% 82±19 vs. 90±22; FVC% 81±15 vs. 91±21). The analysis of paired data revealed a significant growth in FEV1% and FVC% (P<0.001) in both PGD group after 12 months.
Functional CT parameters
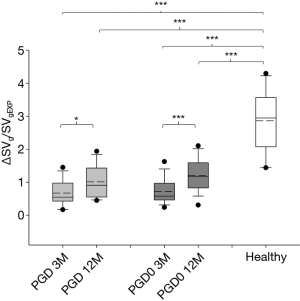
The distribution of ΔSVg/SVgEXP at study time-points is shown in Figure 2. At 3 months, ΔSVg/SVgEXP showed no significant differences among groups. At 12 months, ΔSVg/SVgEXP increased significantly in both groups, in PGD passing from 0.67±0.38 to 1.02±0.50 (P=0.028) and in PGD0 patients from 0.72±0.39 to 1.20±0.49 (P<0.001).
Results from functional mask analysis are reported in Figure 3. The distribution of %LV, %H %C, and %AT at 3 and 12 months post transplantation in PGD and PGD0 groups are showed.
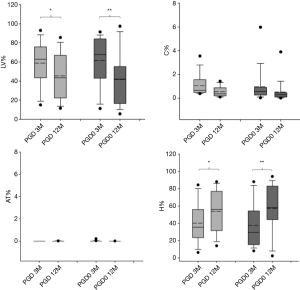
The analysis for paired data revealed that there was a significant decrease in LV% in both PGD group after 12 months (P<0.05 and P<0.01, respectively) and a correspondent significant increase in H% (P<0.05 and P<0.01, respectively). %C and %AT were negligible in both transplanted groups at 3 and 12 months.
Figure 4 reports the values of paired data analysis for ΔSVg/SVgEXP, %LV and %H at 3 and 12 months in PGD and PGD0 groups. A great variability was observed in both groups within the first year after lung transplantation, but there was a significant increase in ΔSVg/SVgEXP value at 12 months in both groups (Figure 4A,B). For the functional mask analysis, both groups demonstrated a significant decrease in the mean value of %LV (Figure 4C,D) and a consequent significant increase in %H (Figure 4E,F).
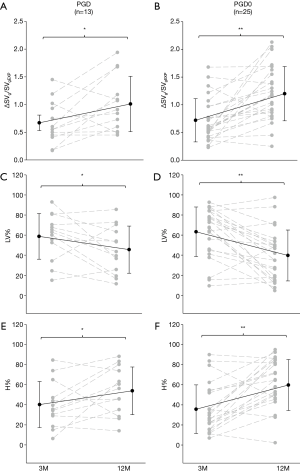
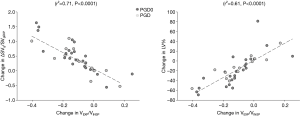
Figure 5 shows CT functional masks at 3 and 12 months from two representative cases belonging to the two transplanted groups. In order to determine the relationship between CT parameters, ΔSVg/SVgEXP and %LV, and VEXP/VINSP correlations were performed in PGD and PGD0 patients (Figure 6). VEXP/VINSP from 3 to 12 months was inversely associated with ΔSVg/SVgEXP (r2=0.71, P<0.0001) and with %LV (r2=0.61, P<0.0001).
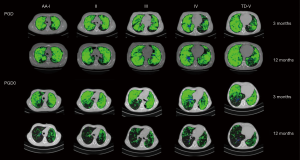
Discussion
Nowadays, lung transplantation is a consolidated approach for the treatment of end-stage respiratory diseases, but still burdened with high rates of complications. Despite technical progress has diminished the early postoperative mortality, the incidence of PGD remains significant; such syndrome is associated to poor early outcome as well as to impaired long-term survival (6,17,18). Why and how the early graft damage can affect the long-term outcome remains to be completely understood. The promising studies with CT scan were occasional and not conclusive (19). In the same manner, although considered a “gold standard”, PFTs are subject to some degree of variability and patient compliance.
In our study, pulmonary function parameters were affected by the onset of PGD only at early time (3 months); at 12 months a decrease of the incidence of the effect of PGD was observed and no differences were found between the two transplanted groups. Furthermore, PFTs do not yield information about regional distribution of ventilation defects. Regional analysis with CT could be an attractive technique to interpret lung patterns after transplantation. In this study, we evaluate the application of CT functional mask derived parameters to determine whether development of severe PGD in the perioperative period is associated with short and/or long-term postoperative evidences of pulmonary function alterations. Quantitative CT regional analysis, proposed in the frame of this work, may provide a significant advance in the interpretation of ventilation abnormalities after lung transplant.
We enrolled patients who underwent lung transplantation in our center and we considered the subjects who completed the 1-year follow-up. To avoid the confounding effect of the native lung on pulmonary function we only selected bilateral recipients with the aim to evaluate the effect of PGD on the ventilation defects as well as their distribution in space and in time. We categorized our population into two groups based on the occurrence of PGD within the first 72 hours after surgery. PGD grade 1 was present in only one patient. We decided to exclude PGD grade 1 from the study for two main reasons: (I) grouping PGD1 with highest grade would had been misleading because, actually, the functional impairment is negligible; on the other hand, (II) grouping PGD1 with patients who did not experience pulmonary infiltrate was illogical. Only an adequate number of patients with PGD1 would allow a targeted analysis.
The major findings of our study are: (I) the significant reduction of the ΔSVg/SVgEXP in PGD and PGD0 patients compared to healthy (8); (II) the significant higher prevalence of low ventilation pattern in PGD and PGD0 groups at 3-month follow-up; (III) the negligible differences in ΔSVg/SVgEXP and %LV among transplanted groups at 3 months, and (IV) the significant higher correlation between the percentage of ΔSVg/SVgEXP and low ventilation and the VEXP/VINSP in both transplanted groups at 3 and 12-month follow-up.
We measured ΔSVg/SVgEXP of the whole lung in order to quantify in a single parameter the degree of lung emptying (numerator) and expiratory lung hyperinflation/air trapping (denominator), providing a significant tool in the interpretation of ventilation abnormalities after lung transplantation. Our results demonstrate a marked decrease in ΔSVg/SVgEXP in all subjects, both at 3 and 12 months after lung transplantation, indicating a high degree of ventilation defects and/or expiratory lung hyperinflation affecting the normal distribution of lung ventilation even in patients with ideal post-transplantation course. In order to better identify the pattern of ventilation defects in the post-transplantation period, we introduced a novel CT-based methodology providing detailed information which could be the basis for future targeted interventions. Despite of PGD grading we found in all the subjects high percentages of low ventilation areas while air trapping or consolidation areas were negligible; once this clinical frame will be confirmed with larger studies, interventions such as non-invasive ventilation could be considered to counterbalance the persisting low ventilation pattern.
A further evidence that the decrease of ΔSVg/SVgEXP in transplanted patients is due more to ventilation defects rather than to air trapping and/or consolidation is provided by the results of Figure 6, showing that a significant, linear correlation between ΔSVg/SVgEXP and both the extent of low ventilation patterns and the decline of the ratio of lung expiratory to inspiratory volumes (VEXP/VINSP) in the first year after lung transplantation.
Our results, therefore, suggest that when multi-volumetric CT scans are not available, in addition to spirometric indices, the measurement of static lung volumes might be useful to identify and differentiate particular physiological phenotypes and predict survival in patients with chronic lung allograft dysfunction as recently reported (20).
The negligible differences in ΔSVg/SVgEXP and %LV among transplanted groups at short and mid-term suggests that a number of confounding factors could play a role in ventilation defects.
Our study has limitations that should be considered when interpreting the results. First, our study was a pilot prospective cohort study with a limited sample size. Furthermore, longer follow-up is necessary to study the chronic lung allograft dysfunction onset and identify the correlation with the PGD syndrome under the view of CT volume analysis.
Conclusions
In summary, we demonstrate that quantification of ventilation defects by CT functional mask can offer insight into the correlation between PGD and pulmonary function after lung transplantation at short and mid-term. Irrespective of whether or not the occurrence of PGD, our results suggest the importance of careful and integrated functional studies as a base to a tailored respiratory physiotherapy aimed at contrasting the persistence of low ventilation areas in the lung after transplantation. Further multi-centric studies using a larger sample size are essential to investigate the usefulness of this novel methodology in monitoring and quantifying the distribution of lung abnormalities in transplanted lungs.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the local ethics committee (749_2016bis) and written informed consent was obtained from all patients.
References
- Christie JD, Bavaria JE, Palevsky HI, et al. Primary graft failure following lung transplantation. Chest 1998;114:51-60. [Crossref] [PubMed]
- Diamond JM, Arcasoy S, Kennedy CC, et al. Report of the International Society for Heart and Lung Transplantation Working Group on Primary Lung Graft Dysfunction, part II: Epidemiology, risk factors, and outcomes-A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2017;36:1104-13. [Crossref] [PubMed]
- Christie JD, Sager JS, Kimmel SE, et al. Impact of primary graft failure on outcomes following lung transplantation. Chest 2005;127:161-5. [Crossref] [PubMed]
- Snell GI, Yusen RD, Weill D, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: Definition and grading-A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2017;36:1097-103. [Crossref] [PubMed]
- Kreisel D, Krupnick AS, Puri V, et al. Short- and long-term outcomes of 1000 adult lung transplant recipients at a single center. J Thorac Cardiovasc Surg 2011;141:215-22. [Crossref] [PubMed]
- Armstrong HF, Lederer DJ, Bacchetta M, et al. Primary graft dysfunction: Long-term physical function outcomes among lung transplant recipients. Heart Lung 2016;45:544-9. [Crossref] [PubMed]
- Belmaati EO, Iversen M, Kofoed KF, et al. Scintigraphy at 3 months after single lung transplantation and observations of primary graft dysfunction and lung function. Interact Cardiovasc Thorac Surg 2012;14:792-6. [Crossref] [PubMed]
- Salito C, Barazzetti L, Woods JC, et al. Heterogeneity of specific gas volume changes: a new tool to plan lung volume reduction in COPD. Chest 2014;146:1554-65. [Crossref] [PubMed]
- Angel E, Yaghmai N, Jude CM, et al. Dose to radiosensitive organs during routine chest CT: effects of tube current modulation. AJR Am J Roentgenol 2009;193:1340-5. [Crossref] [PubMed]
- Aliverti A, Pennati F, Salito C, et al. Regional lung function and heterogeneity of specific gas volume in healthy and emphysematous subjects. Eur Respir J 2013;41:1179-88. [Crossref] [PubMed]
- Coxson HO, Rogers RM, Whittall KP, et al. A quantification of the lung surface area in emphysema using computed tomography. Am J Respir Crit Care Med 1999;159:851-6. [Crossref] [PubMed]
- Salito C, Aliverti A, Gierada DS, et al. Quantification of trapped gas with CT and 3 He MR imaging in a porcine model of isolated airway obstruction. Radiology 2009;253:380-9. [Crossref] [PubMed]
- Salito C, Woods JC, Aliverti A. Influence of CT reconstruction settings on extremely low attenuation values for specific gas volume calculation in severe emphysema. Acad Radiol 2011;18:1277-84. [Crossref] [PubMed]
- Lucas BD, Kanade T. An iterative image registration technique with an application to stereo vision. Proceeding of IJCAI 1981;81:674-9.
- Pennati F, Salito C, Aliverti A. Registration of lung CT images acquired in different respiratory ranges with 4DCT and HRCT. Conf Proc IEEE Eng Med Biol Soc 2015;2015:2936-9. [PubMed]
- Rosenblum LJ, Mauceri RA, Wellenstein DE, et al. Density patterns in the normal lung as determined by computed tomography. Radiology 1980;137:409-16. [Crossref] [PubMed]
- Lee JC, Christie JD, Keshavjee S. Primary graft dysfunction: definition, risk factors, short- and long-term outcomes. Semin Respir Crit Care Med 2010;31:161-71. [Crossref] [PubMed]
- Whitson BA, Prekker ME, Herrington CS, et al. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J Heart Lung Transplant 2007;26:1004-11. [Crossref] [PubMed]
- Belmaati EO, Steffensen I, Jensen C, et al. Radiological patterns of primary graft dysfunction after lung transplantation evaluated by 64-multi-slice computed tomography: a descriptive study. Interact Cardiovasc Thorac Surg 2012;14:785-91. [Crossref] [PubMed]
- Kneidinger N, Milger K, Janitza S, et al. Lung volumes predict survival in patients with chronic lung allograft dysfunction. Eur Respir J 2017;49. [Crossref] [PubMed]