
Evidence for surgical resections in oligometastatic lung cancer
Introduction
Stage IV lung cancer occurs in about 40–50% of patients with non-small cell lung cancer (NSCLC) and about 7% of these patients will show just a solitary or a limited number of metastasis after complete examination. With a poor median survival rate of around 8–11 months, its management historically is limited to palliative chemotherapy. The management of lung cancer has evolved over the past decade, as more knowledge has been acquired with regards to its molecular biology, pathogenesis and response to treatment. The once followed dictum of palliative chemotherapy for metastatic NSCLC has drastically changed over the past few years concurrent with the advancement in diagnostics, and increasing evidence to suggest that these once incurable disease are now potentially curable or at least has favourable outcomes in terms of median survival and disease free survival after control of primary tumor and local ablative therapies are instituted to the metastatic sites.
Oligometastatic state, a clinical spectrum which is often defined as the intermediate stage between locally advanced and widely disseminated disease was first proposed by Hellman and Weichselbaum in 1995, anchoring on the hypothesis that the pathogenesis of cancer is a biologic spectrum or a multi-step process that begins from a localised entity to a systemic disease with many intermediate states (1). Basing on this ideology, an indolent course of the disease provides opportunity to aggressive control and afford good outcome. It is therefore important to recognize that oligometastasis is based on limited state of metastatic capacity and is a characteristic of many tumors in its evolution (1).
The state of oligometastasis
As the concept of oligometastasis is widely gaining popularity among clinicians, several clinical situations can be construed to be an oligometastatic state. Patients who presented with limited metastases at the time of diagnosis, those with multiple metastases who are rendered oligometastatic from an effective response to systemic treatment which eradicated most metastases but failed to destroy one or a limited number of treatment-resistant tumor foci are all within the realm of oligometastasis (1). On the other hand, oligo-recurrence pertains to a condition where the primary site of cancer is controlled, and all gross recurrent or metastatic sites could be treated using local therapy (2). Issues with regards to the number of metastases as to either limited to one, two to three metastases and as many as five have been used. The number of sites involved varies from different studies. Involvement of one to three distant sites was the most commonly used cut-off to satisfy the criteria. Lymph node positivity also contributes a major factor to the outcomes of patients with oligometastasis. These criteria would later on prove to be useful to select patients with oligometastases who are good candidate for aggressive therapy (3).
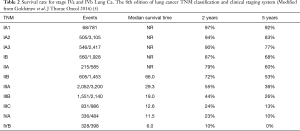
Several literatures support the existence of oligometastasis. In recent years, numerous articles were published for colorectal cancer with liver metastasis having 10-year survival rates of up to 20–26% after treatment to the primary tumor and the liver metastasis was made. The international registry of lung metastases reported the outcomes of 5,206 patients with resected lung metastases from variety of primary cancers and reported a 5-year survival of 36% (4). Furthermore, the 8th edition of Lung Cancer TNM Classification and Clinical staging system reclassified the M Stage descriptor to M1a-M1c (Table 1). This clearly identifies these subset of population with single extra-thoracic metastasis including those with single distant non-regional lymph nodes as M1b. In this context, patients with M1b metastasis is Staged as IVA rather than IVB. More significantly, stage IVA has a better survival rate of 23% and 10% at 24 and 60 months respectively, compared with stage IVB which has 10% and 0% survival rate within the same period of time (5) (Table 2). A systemic review by Ashworth et al. of 49 articles covering a total of 2,176 NSCLC patients who received locally ablative treatment to oligometastases, showed that 25% of the total population studied has variety of metastatic site and 68.9% of all the patient had brain metastasis. Most of the patient had limited metastatic disease while more than half had a solitary metastasis and at least 85% of patients had 3 or fewer metastases. In 7 out of the 49 studies included, showed patients with 1–5 metastases (6). The review clearly demonstrates that a state of oligometastasis is in existence.

Full table
Full table
Prognostic factors, patient selection and treatment
In the same review by Ashworth et al., they were able to group the factors which offer better outcomes for patients with NSCLC oligometastasis. The prognostic factors deemed highly significant are those with controlled primary tumor, a lower primary tumor N-stage and a longer disease-free interval of more than 6–12 months as these variables correlates well with better overall survival (6). Control of primary tumor by means of doing radical or definitive surgery, having complete resection of the primary tumor offers better overall survival. Likewise patients with N0 stage, in 5 of the studies included in the review fared better than those having higher N stage disease. Consequently N0 and N1 disease fared better than N2 or N3 disease. A longer duration of disease-free status also showed favorable overall survival in patients with brain metastases [disease free interval (DFI) >360 days compared to less], multiple metastatic sites (DFI >1 year), and DFI of more than 6 months for those with adrenal metastases (6). Other factors considered moderately significant included presence of extra-cranial metastases as this is associated with a decreased overall survival, use of PET-CT, RPA classification, primary tumor size (primary tumors 1–3 cm fared better compared with those with primary tumors more than 3 cm) and type of resection (6). In a meta-analysis study by Li et al. comprising of 1,935 patients, they noted that female gender (HR 1.21, 95% CI: 1.02–1.45, P=0.03), (y)pN0 stage (HR 1.82, 95% CI: 1.40–2.36, P<0.00001), adenocarcinoma (HR 1.44, 95% CI: 1.10–1.88, P=0.008) showed favourable outcomes (3). In another meta-analysis of 757 patients with oligometastatic NSCLC (one to five metastases) treated with curative intent to all sites of disease, they were able to separate patients with oligometastatic NSCLC into three prognostic groups. Low risk group patients pertains to those with metachronous metastases whom fared best (5-year OS, 47.8%), followed by patients with intermediate risk where the lymph node status is N0 and has synchronous metastases (5-year OS 36.2%), whereas high risk group of patients are those with synchronous metastases and pathologically involved regional LN faring the worst (5-year OS 13%) (7). Bearing in mind these factors would help select oligometastatic patients who will benefit most from treatment.
The idea of instituting management for patients with oligometastases was inspired by a study conducted by Luketich et al. where they identified 14 patients who had solitary extracranial metastases treated aggressively after curative treatment of their NSCLC. In the study, 12 of the 14 patients had complete surgical resection of the metastatic site and 2 had curative irradiation. The study outcome manifested an overall 10-year actuarial survival of 86% (8). In the presence of a well-controlled or completely resectable primary lung cancer and a negative extensive metastasis work-up, surgical removal of a synchronous or metachronous solitary brain metastasis has resulted in 5-year survival rates ranging from 10% to 30% (8). The primary treatment options for definitive therapy of oligometastases are surgery and/or radiation therapy which may also be combined with systemic therapy as well as other ablative modalities such as radio frequency, cryoablation and microwave ablation. In a multi-institutional phase II randomized study by Gomez et al. involving 49 patients aiming to evaluate the effect of local consolidative therapy (surgery, radiotherapy or combined as mode of treatment for both primary and metastatic lesion) followed by standard maintenance or observation (LCT) versus maintenance therapy or observation only for patients with stage IV NSCLC presenting with limited metastases (less than or has 3 disease sites after systemic therapy and no disease progression after randomisation) on progression free survival (PFS), the study showed that the median PFS for patients who underwent LCT was 11.9 months (90% confidence interval) versus 3.9 months in the observation or maintenance group (hazard ratio 0.35; 90% CI: 0.18–0.66, log-rank P=0.0054) (9). The 1-year PFS rate for the LCT arm was 48% compared with 20% for the observational arm. Likewise, the time to the appearance of a new lesion was longer among patients in the LCT arm (11.9 vs. 5.7 months in the no-LCT arm; P=0.0497) (9). Evaluating data from seven retrospective cohort studies, including 668 patients, treated with aggressive thoracic therapy (ATT) (surgery or radiotherapy) was associated with a 52% overall reduction in terms of risk of death. Pooled 1-, 2-, 3-, and 4-year survival rates of patients receiving ATT were 74.9%, 52.1%, 23.0%, and 12.6%, respectively, versus 32.3%, 13.7%, 3.7%, and 2.0%, respectively, for those not receiving ATT (10). At present, the management of patients with NSCLC has been changing rapidly. Most of the surgical literature advocate resection of the primary cancer with either surgical metastasectomy with or without chemotherapy, ablation of the metastatic lesion using radiofrequency, microwave, cryotherapy with or without chemotherapy or stereotactic body radiation therapy (SBRT) with or with chemotherapy (11). With the use of immunotherapy both in the early and late phase of the disease, it has contributed a lot to the paradigm shift in lung cancer treatment. The combination of immunotherapy and SBRT on primary tumor and/or metastatic sites could represent the best approach to treating oligometastatic NSCLC in the near future (12).
Treatment by site
NSCLC with contralateral lung metastasis
The International Registry of Lung Metastases has reported that the 5-year OS for patients with complete resection of metastatic lung tumors was 36% compared to 13% for those without (4). A popular approach in the management of patients with contralateral lung metastasis involved a staged procedure wherein resection of the metastatic tumor with sub-lobar resection is done first followed by a lobectomy or bilobectomy of the primary tumor. The procedure entails a low morbidity and mortality of 0–2.5% (13). SBRT may also be employed for such cases as this modality can achieve local control as high as 92–98% for patients with metastatic lung tumors as reported by Blomgren et al. and Uematsu et al. (14,15). However all of these studies were retrospective in nature. In a phase I/II prospective study of SBRT for metastatic lung tumors involving 38 patients, SBRT achieved a 2-year local control rate of 96% and 2-year OS of 39% (16). The ACCP guidelines of 2013 also recommends that in patients with contralateral lobe nodule, it is suggested that evaluation of extrathoracic metastases (e.g., PET and brain MRI/CT) and invasive evaluation to rule out mediastinal node involvement should be carried out and resection of each lesion is suggested if nodes are negative and patient has adequate pulmonary reserve (Grade 2C).
NSCLC with brain metastasis
The brain is the most common site of distant metastasis for NSCLC. Newly diagnosed patients with NSCLC will have a 25–30% chance of harbouring metastasis to their brain at the time of diagnosis. Brain metastasis from NSCLC carries with it a dismal OS of 2 months with steroid alone and around 6 months with whole brain radiotherapy (WBRT). In a retrospective case series for patients with a solitary synchronous brain metastasis who underwent aggressive treatment of the metastatic lesion and loco-regional treatment to the lung primary showed a median OS ranging from 7 to 24 months, and 5-year survival range of 7–24% (17). The study likewise showed that patients who have N0 disease had a superior 5-year survival compared to those with N positive disease (P=0.001) as this was also associated with survival of more than 2 years (17). Having this information, the importance of adequate lymph node staging should be of utmost concern. The study also showed no statistical difference in the survival outcome between synchronous and metachronous solitary brain metastases. Several randomised trials were made to evaluate the effect of resection of brain metastasis with whole brain radiotherapy (WBRT) and WBRT alone. In two randomised trials involving 111 patients, the OS was 40 weeks for patients with brain metastasis resection with WBRT compared with 15 weeks for WBRT alone (P<0.01), likewise resection with WBRT showed better preserved functional independence, and longer functional deterioration of 38 vs. 8 weeks (P<0.005) (18, 19). The current ACCP guideline recommends that for patients with isolated brain metastases, if considered for curative treatment, invasive mediastinal staging and either whole-body PET or abdominal CT plus bone scan are suggested (Grade 2C) and if negative, and primary tumor completely resected, resection or radiosurgical ablation of brain metastasis is recommended (Grade 1C) after which, adjuvant whole-brain radiotherapy is suggested (Grade 2B) plus adjuvant chemotherapy.
NSCLC with adrenal metastasis
The incidence of adrenal metastasis from NSCLC varies but are usually common. Up to 9% of early stage lung cancer undergoing curative resection have unsuspected adrenal metastasis and approximately 1.6% of patients with otherwise operable NSCLC will have unilateral isolated adrenal metastasis. The caveat in the treatment of patients with NSCLC and isolated adrenal metastasis lies in the pathological diagnosis of the metastasis itself. Diagnosis of benign or malignant adrenal lesion (in presence of NSCLC) by MRI or PET-CT is not accurate. Histological diagnosis therefore should be sought. In retrospective case series; of these subset of population median OS ranges from 11 to 31 months with 5-year survival range of 7–60% (17). Several surgical literatures also offered prognostic factors that contribute to good treatment outcomes of patients with oligometastasis to the adrenal from NSCLC. The presence of synchronous adrenal metastases (usually defined as disease-free interval of less than 6 months from the primary diagnosis of lung carcinoma confers a poorer prognosis compared to metachronous adrenal metastases (20). Retroperitoneal lymphatic passages may allow more “direct” tumor spread from lung to ipsilateral adrenal gland and may be associated with better prognosis compared with contralateral metastases (21). In a retrospective series of 20 patients who underwent lung resection and adrenalectomy for oligometastatic NSCLC, 7 patients (35%) with ipsilateral adrenal metastasis and 13 patients (65%) contralateral adrenal metastases, interestingly, ipsilateral group showed a 5-year survival of 83% versus 0% for contralateral metastases (P=0.003) (22). The current ACCP guideline for treatment of patients with adrenal metastases recommend that if patients are for curative-intent surgical resection, invasive mediastinal staging and head CT/MRI plus either whole-body PET or abdominal CT plus bone scan are suggested (Grade 2C). Synchronous metastases with resectable N0 or N1 NSCLC and no other sites of metastases, resection of the primary tumor and the metastasis is recommended (Grade 1C). Metachronous lesions in patients with no other sites of metastases and a previously completely resected primary NSCLC (metachronous presentation), resection of an isolated adrenal metastasis is recommended (Grade 1C), and in patients who have undergone a curative resection of an isolated adrenal metastasis, adjuvant chemotherapy is suggested (Grade 2B).
Conclusions
Pulmonary resection of primary lung CA and the oligometastases should be considered in patients with good performance status and adequate lung function. Apart from whole body and organ specific imaging needed to exclude advanced metastatic disease, an adequate and intensive invasive mediastinal LN staging (EBUS/mediastinoscopy) is recommended in patients being considered for curative approach as higher N staging is associated with worse outcomes. A good evaluation of the prognostic factors as mentioned can provide guidance towards selecting the best candidates for treatment. Curative intent for oligometastatic disease to organs other than lung, brain, adrenal should be considered in a case to case basis. The incorporation and implementation of new drugs such as immunotherapy and tyrosine kinase inhibitor therapy in the multimodal treatment regimens contributes to good treatment outcome of these patients. Likewise an optimal follow-up strategy is crucial, aimed at early detection of oligo-recurrence.
Acknowledgements
None.
Footnote
Conflicts of Interest: Calvin S. H. Ng is a consultant for Johnson and Johnson and Medtronic, USA. Other authors have no conflicts of interest to declare.
References
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Niibe Y, Hayakawa K. Oligometastases and Oligo-recurrence: The New Era of Cancer Therapy. Jpn J Clin Oncol 2010;40:107-111. [Crossref] [PubMed]
- Li S, Zhu R, Li D, et al. Prognostic factors of oligometastatic non-small cell lung cancer: a meta-analysis. J Thorac Dis 2018;10:3701-13. [Crossref] [PubMed]
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions. International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Ashworth A, Rodrigues G, Boldt G, et al. Is there an Oligometastatic state in non-small cell lung cancer? A systemic review of the literature. Lung Cancer 2013;82:197-203. [Crossref] [PubMed]
- Ashworth AB, Senan S, Palma DA, et al. An individual patient data meta analysis of outcomes and prognostic factors after treatment of oligometastatic non-small cell lung cancer. Clin Lung Cancer 2014;15:346-55. [Crossref] [PubMed]
- Luketich JD, Martini N, Ginsberg R, et al. Successful Treatment of Solitary Extracranial Metastases From Non-Small Cell Lung Cancer. Ann Thorac Surg 1995;60:1609-11. [Crossref] [PubMed]
- Gomez DR, Blumenschein G, Lee J, et al. Local Consolidative Therapy versus Maintenance Therapy/Observation for Patients with Oligometastatic Non-Small Cell Lung Cancer without Progression after Front-Line Systemic Therapy: Results of a Multi-Institutional Phase II Randomized Study. Lancet Oncol 2016;17:1672-82. [Crossref] [PubMed]
- Li D, Zhu X, Wang H, et al. Should aggressive thoracic therapy be performed in patients with synchronous oligometastatic non-small cell lung cancer? A meta-analysis. J Thorac Dis 2017;9:310-7. [Crossref] [PubMed]
- Sulaiman NS, Fujii O, Demizu Y, et al. Particle beam radiation therapy using carbon ions and protons for oligometastatic lung tumors. Radiat Oncol 2014;9:183. [Crossref] [PubMed]
- Reynders K, Illidge T, Siva S, et al. Abscopal effect of local radiotherapy using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev 2015;41:503-10. [Crossref] [PubMed]
- Mordant P, Rivera C, Legras A, et al. Current Readings: The Most Influential and Recent Studies Regarding Resection of Lung Cancer in M1a Disease. Semin Thorac Cardiovasc Surg 2013;25:251-5. [Crossref] [PubMed]
- Blomgren H, Lax I, Näslund I, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol 1995;34:861-70. [Crossref] [PubMed]
- Uematsu M, Shioda A, Tahara K, et al. Focal, high dose, and fractionated modified stereotactic radiation therapy for lung carcinoma patients: a preliminary experience. Cancer 1998;82:1062-70. [Crossref] [PubMed]
- Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol 2009;27:1579-84. [Crossref] [PubMed]
- Villaruz LC, Kubicek GJ, Socinski MA. Management of non-small cell lung cancer with oligometastasis. Curr Oncol Rep 2012;14:333-41. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990;322:494-500. [Crossref] [PubMed]
- Vecht CJ, Haaxma-Reiche H, Noordijk EM, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol 1993;33:583-90. [Crossref] [PubMed]
- Tanvetyanon T, Robinson LA, Schell MJ, et al. Outcomes of adrenalectomy for isolated synchronous v metachronous adrenal metastases in non-small cell lung cancer: a systematic review and pooled analysis. J Clin Oncol 2008;26:1142-7. [Crossref] [PubMed]
- Meyer KK. Direct lymphatic connections from the lower lobes of the lung to the abdomen. J Thorac Surg 1958;35:726-33. [PubMed]
- Raz DJ, Lanuti M, Gaissert HC, et al. Outcomes of patients with isolated adrenal metastasis from non-small cell lung carcinoma. Ann Thorac Surg 2011;92:1788-92. [Crossref] [PubMed]


