
The role of the immune system in lung transplantation: towards improved long-term results
History of lung transplantation and introduction
Lung transplantation is a relatively young field. The first human lung transplant was performed in 1963 at the University of Mississippi (1). The recipient had emphysema and lung cancer obstructing the left mainstem bronchus and nephrotic syndrome. He underwent a left single lung transplant and was treated with mediastinal radiation, azathioprine, and steroids for immunosuppression. Post-operatively, he was weaned from ventilatory support, but died 18 days later due to complications of renal failure. Post-mortem examination of the lung showed no evidence of rejection. In spite of the poor outcome, this case illustrated that a single transplanted lung would function, and that rejection could be averted at least for a brief time with the available immunosuppression at the time (1). Over the ensuing decade, 36 additional lung transplants were reported worldwide, but outcomes were uniformly poor (2). Many patients were moribund at the time of transplantation and would not be considered candidates today. Pneumonia, rejection, and respiratory failure were common causes of death, and there were no long-term survivors. Growing experience in heart and heart-lung transplantation paved the way for refinements in surgical techniques of lung transplantation. Additionally, the advent of cyclosporine and encouraging experience with its use in kidney transplantation facilitated the rebirth of lung transplantation (3,4). In 1983, the Toronto Lung Transplant Group performed the first successful lung transplant, and the results were reported as part of a two-patient series in 1986 (5). This was the beginning of lung transplantation in the modern era, and volumes rapidly increased as lung transplantation emerged as the ultimate treatment for end-stage lung disease. In the latest International Society for Heart and Lung Transplantation (ISHLT) Registry report, over 4,600 lung transplants were performed in adults and children in 2016 (6). The indications span the spectrum of lung disease; interstitial lung disease (ILD), chronic obstructive pulmonary disease (COPD), and cystic fibrosis (CF) comprise approximately 80% of underlying diagnoses leading to transplant (6). Over the past 15 years, the volume of bilateral transplants has increased, and in 2016, 80% of all lung transplants reported to the ISHLT Registry were bilateral (6). Indeed, bilateral transplantation is associated with better long-term survival (6).
Lung transplantation improves survival and quality of life when timed appropriately (7-11). Furthermore, there has been a significant improvement in survival after lung transplantation over time, and the median survival in the most recent era between 2009 and 2016 is 6.5 years (6). However, survival after lung transplantation remains significantly worse than after kidney, heart, and liver transplantation (12-14). Early after lung transplantation, infection and allograft failure due to primary graft dysfunction (PGD) are the leading causes of death; however, chronic rejection, termed chronic lung allograft dysfunction (CLAD), is the primary cause of death beyond the first year after transplantation, accounting for approximately 40–50% of deaths (6). Infection remains a significant problem at all time points, and according to the ISHLT Registry, 15–20% of deaths beyond the first year are due to infection (6). Thus, chronic rejection and infection account for up to 70% of deaths beyond the first year after lung transplantation. This underscores the critical role of the immune system and the delicate balance of appropriate immunosuppression after lung transplantation. More intensive immunosuppression may increase the risk and severity of infection and malignancy while less intensive immunosuppression increases the risk of rejection. Indeed, the inability to accurately gauge the degree of immunosuppression has been a significant challenge in clinical practice as the current approach of targeting various calcineurin inhibitor (CNI) trough levels or steroid or cell-cycle inhibitor dose is grossly imprecise.
Rejection after lung transplantation
Hyperacute rejection
Different forms of lung rejection exist, and an overview of these is presented here. Hyperacute rejection is a fulminant form of lung rejection that occurs within minutes or hours of reperfusion of the allograft (15-18). In clinical practice, distinguishing hyperacute rejection from severe PGD can be difficult, and the results of histocompatibility testing are critical to establishing the diagnosis. Pre-formed donor-specific antibodies (DSA) cause hyperacute rejection. DSA are most commonly directed at mismatched human leukocyte antigens (HLA) although non-HLA antibodies may also cause hyperacute rejection (19). The paradigm for the pathogenesis of hyperacute rejection is that DSA bind HLA molecules on endothelial cells and activate the complement cascade which results in endothelial cell necrosis, exposure of the basement membrane, activation of the coagulation cascade and hemorrhagic infarction. Over the past 10 years, hyperacute rejection has become rare because of advances in HLA antibody detection methods. Indeed, solid phase assays have significantly improved the sensitivity and specificity of HLA antibody detection before transplantation (20,21). This allows transplant centers to identify unacceptable antigens for a sensitized patient on the waiting list and minimizes the possibility of a positive crossmatch and hyperacute rejection by avoiding the reactive HLA in a potential donor. Nevertheless, although hyperacute rejection has become quite rare, it demonstrates that antibodies can cause fulminant allograft rejection and that the capillary endothelium is the focal point of injury.
Acute cellular rejection (ACR)
In contrast, ACR is a common complication after lung transplantation. In the ISHLT Registry, approximately 30% of adult lung transplant recipients experience at least 1 episode of ACR in the first year after transplantation (6). However, large international registries may underestimate the incidence of ACR because of reporting limitations. In fact, some randomized controlled trials comparing the efficacy of different immunosuppressive agents have reported an incidence of ACR that ranges between 40–50% (22,23). The highest incidence of ACR is in the first 6 months after transplantation. Non-invasive approaches to the diagnosis of ACR including imaging studies and pulmonary function tests are insensitive and typically non-specific. Consequently, transbronchial lung biopsy remains the gold standard for the diagnosis of ACR although the procedure is invasive and carries some risk of complications. According to the standard ISHLT definition of ACR, the characteristic histologic finding is the presence of a mononuclear cell infiltrate circumferentially surrounding small vessels (24-26). The severity of ACR is based on the intensity of infiltration and extension into the adjacent interstitium. Minimal ACR (grade A1) is characterized by scattered perivascular infiltrates that are not easily visible at low magnification. The perivascular infiltrates are identifiable at low magnification in mild ACR (grade A2), and the infiltrate comprises activated lymphocytes, macrophages, and eosinophils that expand the vascular adventitia (Figure 1). Extension of the mononuclear cell infiltrate into the adjacent interstitium and frequent, obvious infiltrates at low magnification are characteristics of moderate ACR (grade A3). Severe ACR (grade A4) is very rare, and is characterized by diffuse infiltrates with necrotizing vasculitis and diffuse alveolar damage.
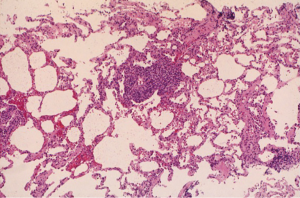
Lower grades of ACR (e.g., A1 and A2) are typically clinically silent, and some cases of moderate ACR (grade A3) are asymptomatic. As a result, many transplant centers employ a surveillance bronchoscopy and transbronchial lung biopsy protocol to identify cases of ACR (27,28). There is no consensus on the utility of surveillance biopsies, and many centers advocate biopsies only if patients develop signs or symptoms of allograft dysfunction (29). Proponents of a surveillance protocol argue that early treatment of ACR may decrease the likelihood of higher grades of ACR or the development of CLAD although empirical evidence to support this is lacking. Nevertheless, historical data suggest that programs that do not use a surveillance protocol may perform an equivalent number of procedures because the threshold to pursue a clinically indicated biopsy may be lower (30).
There is little data about the natural history of ACR. In an early study, asymptomatic patients with mild ACR (grade A2) who were not treated were followed clinically (31). Ten of 16 patients worsened: 4 had persistent A2 rejection, 4 progressed to A3 rejection, 1 developed obliterative bronchiolitis (OB), and 1 developed severe lymphocytic bronchiolitis (31). In addition, 5 of these 10 developed bronchiolitis obliterans syndrome (BOS) during the study follow-up (31). As a result, most patients with mild ACR (grade A2) are treated with bolus methylprednisolone. Furthermore, multiple studies have identified a significant association between ACR and the subsequent development of BOS (32-35). Indeed, even episodes of minimal ACR (grade A1) are associated with an increased risk of BOS (33-35). Nevertheless, although these studies have identified an association between ACR and BOS, this should not suggest that ACR causes BOS. Indeed, it is unclear how the perivascular inflammation characteristic of ACR would result in small airway fibrosis. It is possible that ACR is a marker of the underlying alloimmune response that causes BOS.
Lymphocytic bronchiolitis
Lymphocytic bronchiolitis is characterized by peribronchiolar mononuclear cell infiltrates (26). This airway inflammation often accompanies higher grades of ACR and is considered airway directed acute rejection if there are no signs of a superimposed infection. The ISHLT working formulation for lung allograft rejection defines lymphocytic bronchiolitis grade B1R as submucosal peribronchiolar mononuclear cell infiltrates without epithelial damage or intra-epithelial lymphocytic infiltration (26). Grade B2R is defined as more intense inflammation with activated mononuclear cells including eosinophils and there is intra-epithelial infiltration and epithelial ulceration or necrosis (26). Previous studies have noted that lymphocytic bronchiolitis can be refractory to steroid therapy and is associated with an increased risk of BOS development and death (36,37). The evolution of epithelial ulceration and necrosis to fibrosis and luminal obliteration characteristic of OB is a reasonable explanation for this association. With this in mind, undiagnosed concomitant lymphocytic bronchiolitis may be the explanation for the association between ACR and BOS.
Antibody-mediated rejection (AMR)
AMR is an increasingly recognized form of lung allograft rejection. In recent years, multiple case reports and case series from different centers describing the presentation and clinical features of AMR have been published (38-45). Based on these findings and experience in kidney transplantation, the ISHLT developed a consensus definition for pulmonary AMR (46). According to this definition, the diagnosis of definite AMR is made if all of the following criteria are present:
- Clinical allograft dysfunction;
- Lung injury pathology;
- Capillary C4d deposition;
- Circulating DSA;
- Clinical exclusion of other possible causes of allograft dysfunction.
The diagnostic certainty is dependent on the number of criteria present. If one of the above criteria is absent, the diagnosis of AMR is considered probable, and if 2 of the above criteria are absent, the diagnosis is considered possible (46). However, because C4d staining and interpretation have been problematic in lung transplantation, the committee noted that a diagnosis of AMR can be confidently made in the absence of C4d deposition if all other criteria are present.
This ISHLT definition for pulmonary AMR will facilitate future research and standardize the diagnosis across centers. However, the definition is complex and relies on a multi-disciplinary approach. Indeed, the diagnosis of AMR remains a difficult one and requires a high index of suspicion. It is important to note that the histologic features are typically non-specific. These may include acute and organizing lung injury, pneumonitis, diffuse alveolar damage, capillaritis, and ACR. In the right clinical setting, neutrophilic capillaritis raises the suspicion for AMR (47,48). The characteristic findings are neutrophilic infiltration with karyorrhectic debris in alveolar septa (Figure 2). However, neutrophilic capillaritis is not a sensitive finding. Although C4d deposition provides direct immunopathologic evidence of the effect of antibodies, many cases that have all other criteria are C4d-negative (43,45,48). Indeed, a recent relatively large single center study compared the clinical presentation, DSA characteristics, histologic findings, and outcomes of C4d-positive cases to C4d-negative cases of AMR (48). There were no significant differences between the two groups with the exception that C4d-negative cases were more likely to be due to non-complement binding DSA (48). The authors proposed that C4d-negative cases of AMR be considered definite AMR if all other criteria are present. In addition, they suggested that some C4d-negative cases might be due to complement-independent pathways (48). Furthermore, C4d-negative AMR is now a widely recognized form of AMR in kidney transplantation (49,50). The diagnosis of AMR remains difficult because of the absence of specific histologic findings and the inconsistencies of C4d staining. Confounding matters further, DSA are common but do not necessarily lead to AMR, and there are various causes of allograft dysfunction. Clearly, better diagnostics are necessary to facilitate the identification of AMR. Finally, although AMR may be a reversible form of allograft failure, there is a high incidence of CLAD among survivors (42-45,48).
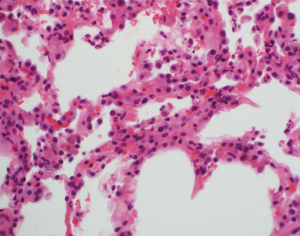
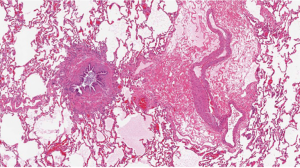
Chronic lung allograft dysfunction
As noted above, CLAD is the leading cause of death beyond the first year after lung transplantation (6). CLAD is stratified into two phenotypes with potential overlap between these. BOS is the prototypic form of CLAD and was recognized in the late 1980s as the critical barrier to better long-term outcomes after heart-lung and lung transplantation (51-54). OB, a fibroproliferative scarring of membranous and respiratory bronchioles that results in luminal obliteration, is the characteristic histology of BOS (Figure 3). However, the sensitivity of transbronchial lung biopsy for the diagnosis of OB is poor because of the small sample size and the patchy nature of OB. As a result, BOS is the clinical surrogate for OB and is defined based on obstructive changes in spirometry with progressive stages defined according to the magnitude of decrease from baseline (53,54). Restrictive allograft syndrome (RAS) has been recognized over the past 10–15 years as a more rapidly progressive form of CLAD (55-58). There are significant differences in spirometry and imaging studies between RAS and BOS. BOS is characterized by an obstructive ventilatory defect whereas RAS manifests with a restrictive ventilatory abnormality. A challenge to the diagnosis of RAS is that measuring total lung capacity (TLC) has not been a part of routine pulmonary function testing at most transplant centers. Thus, baseline values are not available for comparison at disease onset. The pathology of RAS has not been extensively studied. In one series of 16 patients with RAS who had available pathology specimens, 15 had pleuroparenchymal fibroelastosis and 1 had diffuse alveolar damage (59). It is noteworthy that 14 of the 16 patients had concomitant OB (59). This raises questions about whether RAS is truly a distinct and unique phenotype of CLAD or whether it represents a more advanced or severe form. Furthermore, in the original descriptions of OB as chronic lung allograft rejection after heart-lung transplantation, diffuse interstitial and pleural fibrosis as well as a concomitant restrictive ventilatory abnormality were noted (60,61).
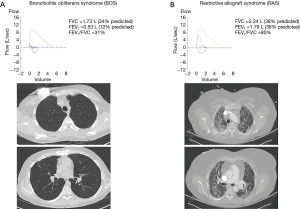
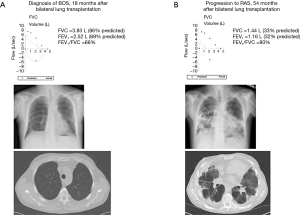
Patients with BOS typically have no abnormalities on chest X-ray or chest computed tomography (CT) until advanced stages where mosaic attenuation, bronchial dilation, or air trapping may appear (62). In contrast, upper lobe predominant coarse radiographic opacities on chest X-ray and CT scan are characteristic of RAS (55,57,58). Figure 4 illustrates differences in spirometry and CT scan findings between a patient with advanced BOS (Figure 4A) and a patient with advanced RAS (Figure 4B). A clinical presentation with features of both BOS and RAS is possible. In addition, some patients may present with BOS at the onset of CLAD diagnosis and evolve into RAS and vice versa (57). Figure 5 illustrates an example of a patient who progressed from BOS to RAS over time. The patient developed BOS 18 months after bilateral lung transplantation (Figure 5A). In spite of intensive treatment, CLAD progressed and by 54 months after transplantation, he had advanced RAS (Figure 5B). At autopsy, the patient was noted to have extensive OB, interstitial fibrosis, and mild ACR.
Evidence-based treatment options for CLAD are limited. There has been only one randomized controlled trial for the management of CLAD where 48 patients with BOS were randomized to azithromycin or placebo (63). In this study, five patients who were randomized to placebo crossed over to open label azithromycin, and there was no significant difference between the azithromycin and the placebo group in the intention to treat analysis (63). However, among those who completed the study, azithromycin was associated with improved lung function compared to placebo (63). Beyond azithromycin, treatment options include bolus methylprednisolone, anti-thymocyte globulin, alemtuzumab, and extracorporeal photopheresis (64-69). In spite of aggressive treatment, the clinical course is typically progressive resulting in respiratory failure and death or re-transplantation in the majority of patients. The median survival after the diagnosis of BOS is approximately 2.5 years (70). However, survival after the diagnosis of RAS is significantly worse (57). Clearly, improved prevention and treatment of CLAD are necessary to improve patient outcomes after lung transplantation.
Infection
Lung transplant recipients are at increased risk of infection at all time points, and infection is a common cause of death accounting for up to 20% of all deaths beyond the first year after transplantation (6). Therapeutic immunosuppression is necessary to mitigate the risk of rejection, but this inherently increases the risk of infection. Furthermore, local host defenses are impaired with the loss of lymphatic drainage and impaired mucociliary clearance and cough mechanism after lung transplantation. Lastly, the lung allograft is in constant contact with the environment, and this increases the risk of acquiring infections. In the immediate period after transplantation, donor-transmitted, recipient-derived, and nosocomial infections are most common. Typical donor-transmitted infections are bacterial pneumonia and community-acquired respiratory viral (CARV) infections. Mycobacterial and endemic fungal infections are less common, and systemic viral infections (e.g., human immunodeficiency virus, hepatitis C virus) are exceedingly rare with current donor testing protocols. Common nosocomial infections include bacterial pneumonia, surgical site infection, empyema, and Clostridium difficile colitis. In general, patients are treated with empiric broad-spectrum antibacterial antibiotics for the first 7–14 days after transplantation, and the choice of agents is adjusted based on donor and recipient culture results.
The risk of opportunistic infections is highest in the first 6 months after transplantation. The risk of cytomegalovirus (CMV) infection depends on the serologic status of the donor and the recipient, and seronegative recipients of organs from seropositive donors have the highest risk. Transplant programs use different prophylactic regimens to prevent CMV infection. In a multicenter randomized controlled trial, extended prophylaxis with valganciclovir to 12 months after transplantation was associated with a significantly lower incidence of CMV disease, CMV infection, and disease severity compared to 3 months of prophylaxis (71). Other prophylactic regimens have not been as carefully studied, but most patients are treated with an antibiotic for prophylaxis against Pneumocystis jiroveci pneumonia. Recipient-derived infections remain common in the first 6 months. In addition, community-acquired infections including CARV (e.g., influenza, respiratory syncytial virus, etc.), bacterial pneumonia and endemic fungi (e.g., histoplasmosis, coccidioidomycosis) can be a significant cause of morbidity.
Infections can have an immediate and direct impact on lung transplant recipients resulting in hospitalization and increased health care utilization (72). Furthermore, multiple infections have been associated with an increased risk of CLAD development and progression in the ensuing months after the infection (72-74). Respiratory viral infections have been linked to the development of BOS (73). The development of epithelial fibrosis and luminal obliteration characteristic of OB after viral bronchiolitis is easy to envision. In addition, bacterial respiratory infections including Staphylococcus aureus and Pseudomonas aeruginosa and fungal colonization with Aspergillus species have been linked to CLAD and increased mortality (74-78). The relationship between the isolation of Pseudomonas aeruginosa and CLAD is more complex. In a large single center study, de novo acquisition of Pseudomonas aeruginosa was associated with an increased risk of CLAD, but the persistence of pre-transplant Pseudomonas aeruginosa culture positivity post-transplant was not (79). A paradigm for the association between infections and the development of CLAD is that organisms stimulate the release of chemokines from the allograft resulting in the recruitment of leukocytes which further amplify the recruitment of additional inflammatory cells and allograft injury (80). It is also possible that alloimmune responses injure the airway epithelium first, and this increases the risk of infection.
Conclusions
Lung transplantation is the ultimate treatment for patients with advanced lung disease. Although there have been significant improvements in survival since lung transplantation became a clinically viable treatment in the 1980s, survival after lung transplantation continues to lag behind survival after other solid organ transplants. Indeed, long-term outcomes remain disappointing in spite of advances in donor and recipient selection and management. Rejection and infection are the leading causes of death after transplantation. This highlights the critical role of the immune response after transplant and underscores the need for better clinical immunosuppression and immune monitoring. Clearly, there are ongoing unmet needs in the management of lung transplant recipients, and future studies are necessary to continue to advance the field and improve patient outcomes.
Acknowledgments
None.
Footnote
Conflicts of Interest: The author has received grant funding from Bristol Myers Squibb and Therakos, is a consultant for Transmedics, and has served on an advisory board for Theravance Biotherapeutics and Vectura.
References
- Hardy JD, Watts RW, Dalton ML, et al. Lung homotransplantations in man. Report of the initial case. JAMA 1963;186:1065-74. [Crossref] [PubMed]
- Veith FJ, Koerner SK. Problems in the management of lung transplant recipients. Vasc Surg 1974;8:273-82. [PubMed]
- Calne RY, White DJ, Thiru S, et al. Cyclosporin A in clinical kidney grafting from cadaver donors. Proc Eur Dial Transplant Assoc 1979;16:305-9. [PubMed]
- Fernando ON, Sweny P, Farrington K, et al. Preliminary experience with cyclosporine A in human renal allografts. Transplant Proc 1980;12:244-5. [PubMed]
- Toronto Lung Transplant Group. Unilateral lung transplantation for pulmonary fibrosis. N Engl J Med 1986;314:1140-5. [Crossref] [PubMed]
- Chambers DC, Cherikh WS, Goldfarb SB, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth adult lung and heart-lung transplant report-2018; Focus theme: Multiorgan Transplantation. J Heart Lung Transplant 2018;37:1169-83. [Crossref] [PubMed]
- Charman SC, Sharples LD, McNeil KD, et al. Assessment of survival benefit after lung transplantation by patient diagnosis. J Heart Lung Transplant 2002;21:226-32. [Crossref] [PubMed]
- De Meester J, Smits JM, Persijn GG, et al. Listing for lung transplantation: Life expectancy and transplant effect, stratified by type of end-stage lung disease, the Eurotransplant experience. J Heart Lung Transplant 2001;20:518-24. [Crossref] [PubMed]
- Hosenpud JD, Bennett LE, Keck BM, et al. Effect of diagnosis on survival benefit of lung transplantation for end-stage lung disease. Lancet 1998;351:24-7. [Crossref] [PubMed]
- Singer JP, Singer LG. Quality of life in lung transplantation. Semin Respir Crit Care Med 2013;34:421-30. [Crossref] [PubMed]
- Singer LG, Chowdhury NA, Faughnan ME, et al. Effects of recipient age and diagnosis on health-related quality of life benefit of lung transplantation. Am J Respir Crit Care Med 2015;192:965-73. [Crossref] [PubMed]
- Wang JH, Skeans MA, Israni AK. Current status of kidney transplant outcomes: Dying to survive. Adv Chronic Kidney Dis 2016;23:281-6. [Crossref] [PubMed]
- Khush KK, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth Adult Heart Transplantation Report-2018; Focus Theme: Multiorgan Transplantation. J Heart Lung Transplant 2018;37:1155-68. [Crossref] [PubMed]
- Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2015 Annual Data Report: Liver. Am J Transplant 2017;17 Suppl 1:174-251. [Crossref] [PubMed]
- Frost AE, Jammal CT, Cagle PT. Hyperacute rejection following lung transplantation. Chest 1996;110:559-62. [Crossref] [PubMed]
- Bittner HB, Dunitz J, Hertz M, et al. Hyperacute rejection in single lung transplantation – case report of successful management by means of plasmapheresis and antithymocyte globulin treatment. Transplantation 2001;71:649-51. [Crossref] [PubMed]
- Masson E, Stern M, Chabod J, et al. Hyperacute rejection after lung transplantation caused by undetected low-titer anti-HLA antibodies. J Heart Lung Transplant 2007;26:642-5. [Crossref] [PubMed]
- Dawson KL, Parulekar A, Seethamraju H. Treatment of hyperacute antibody-mediated lung allograft rejection with eculizumab. J Heart Lung Transplant 2012;31:1325-6. [Crossref] [PubMed]
- Fernandez R, Chiu S, Raparia K, et al. Humoral Human Lung Allograft Rejection by Tissue-Restricted Non-HLA Antibodies. Ann Thorac Surg 2016;102:e339-41. [Crossref] [PubMed]
- Tait BD, Süsal C, Gebel HM, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation 2013;95:19-47. [Crossref] [PubMed]
- Tambur AR, Campbell P, Claas FH, et al. Sensitization in Transplantation: Assessment of Risk (STAR) 2017 Working Group Meeting Report. Am J Transplant 2018;18:1604-14. [Crossref] [PubMed]
- Glanville AR, Aboyoun C, Klepetko W, et al. Three-year results of an investigator-driven multicenter, international, randomized open-label de novo trial to prevent BOS after lung transplantation. J Heart Lung Transplant 2015;34:16-25. [Crossref] [PubMed]
- Hachem RR, Yusen RD, Chakinala MM, et al. A randomized controlled trial of tacrolimus versus cyclosporine after lung transplantation. J Heart Lung Transplant 2007;26:1012-8. [Crossref] [PubMed]
- Berry GJ, Brunt EM, Chamberlain D, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Lung Rejection Study Group. The International Society for Heart Transplantation. J Heart Transplant 1990;9:593-601. [PubMed]
- Yousem SA, Berry GJ, Cagle PT, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection. J Heart Lung Transplant 1996;15:1-15. [PubMed]
- Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant 2007;26:1229-42. [Crossref] [PubMed]
- Kukafka DS, O’Brien GM, Furukawa S, Criner GJ. Surveillance bronchoscopy in lung transplant recipients. Chest 1997;111:377-81. [Crossref] [PubMed]
- Swanson SJ, Mentzer SJ, Reilly JJ, et al. Surveillance transbronchial lung biopsies: Implication for survival after lung transplantation. J Thorac Cardiovasc Surg 2000;119:27-37. [Crossref] [PubMed]
- Valentine VG, Taylor DE, Dhillon GS, et al. Success of lung transplantation without surveillance bronchoscopy. J Heart Lung Transplant 2002;21:319-26. [Crossref] [PubMed]
- Tamm M, Sharples LD, Higenbottam TW, et al. Bronchiolitis obliterans syndrome in heart-lung transplantation – Surveillance biopsies. Am J Respir Crit Care Med 1997;155:1705-10. [Crossref] [PubMed]
- Yousem SA. Significance of clinically silent untreated mild acute cellular rejection in lung allograft recipients. Hum Pathol 1996;27:269-73. [Crossref] [PubMed]
- Burton CM, Iversen M, Carlsen J, et al. Acute cellular rejection is a risk factor for bronchiolitis obliterans syndrome independent of post-transplant baseline FEV1. J Heart Lung Transplant 2009;28:888-93. [Crossref] [PubMed]
- Hopkins PM, Aboyoun CL, Chhajed PN, et al. Association of minimal rejection in lung transplant recipients with obliterative bronchiolitis. Am J Respir Crit Care Med 2004;170:1022-6. [Crossref] [PubMed]
- Khalifah AP, Hachem RR, Chakinala MM, et al. Minimal acute rejection after lung transplantation: A risk for bronchiolitis obliterans syndrome. Am J Transplant 2005;5:2022-30. [Crossref] [PubMed]
- Hachem RR, Khalifah AP, Chakinala MM, et al. The significance of a single episode of minimal acute rejection after lung transplantation. Transplantation 2005;80:1406-13. [Crossref] [PubMed]
- Ross DJ, Marchevsky A, Kramer M, et al. “Refractoriness” of airflow obstruction associated with isolated lymphocytic bronchiolitis/bronchitis in pulmonary allografts. J Heart Lung Transplant 1997;16:832-8. [PubMed]
- Glanville AR, Aboyoun CL, Havryk A, et al. Severity of lymphocytic bronchiolitis predicts long-term outcome after lung transplantation. Am J Respir Crit Care Med 2008;177:1033-40. [Crossref] [PubMed]
- Astor TL, Weill D, Cool C, et al. Pulmonary capillaritis in lung transplant recipients: Treatment and effect on allograft function. J Heart Lung Transplant 2005;24:2091-7. [Crossref] [PubMed]
- Morrell MR, Patterson GA, Trulock EP, et al. Acute antibody-mediated rejection after lung transplantation. J Heart Lung Transplant 2009;28:96-100. [Crossref] [PubMed]
- Astor TL, Galantowicz M, Phillips A, et al. Pulmonary capillaritis as a manifestation of acute humoral allograft rejection following infant lung transplantation. Am J Transplant 2009;9:409-12. [Crossref] [PubMed]
- Lobo LJ, Aris RM, Schmitz J, et al. Donor-specific antibodies are associated with antibody-mediated rejection, acute cellular rejection, bronchiolitis obliterans syndrome, and cystic fibrosis after lung transplantation. J Heart Lung Transplant 2013;32:70-7. [Crossref] [PubMed]
- Witt CA, Gaut JP, Yusen RD, et al. Acute antibody-mediated rejection after lung transplantation. J Heart Lung Transplant 2013;32:1034-40. [Crossref] [PubMed]
- Otani S, Davis AK, Cantwell L, et al. Evolving experience of treating antibody-mediated rejection following lung transplantation. Transpl Immunol 2014;31:75-80. [Crossref] [PubMed]
- Roux A, Bendib Le Lan I, Holifanjaniaina S, et al. Antibody-mediated rejection in lung transplantation: Clinical outcomes and donor specific antibody characteristics. Am J Transplant 2016;16:1216-28. [Crossref] [PubMed]
- Ensor CR, Yousem SA, Marrai M, et al. Proteasome inhibitor carfilzomib-based therapy for antibody-mediated rejection of the pulmonary allograft: Use and short-term findings. Am J Transplant 2017;17:1380-8. [Crossref] [PubMed]
- Levine DJ, Glanville AR, Aboyoun C, et al. Antibody-mediated rejection of the lung: A consensus report of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2016;35:397-406. [Crossref] [PubMed]
- Yousem SA, Zeevi A. The histology of lung allograft dysfunction associated with the development of donor-specific HLA antibodies. Am J Surg Pathol 2012;36:987-92. [Crossref] [PubMed]
- Aguilar PR, Carpenter D, Ritter J, et al. The role of C4d deposition in the diagnosis of antibody-mediated rejection after lung transplantation. Am J Transplant 2018;18:936-44. [Crossref] [PubMed]
- Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: inclusion of C4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 2014;14:272-83. Erratum in: Erratum. [Am J Transplant 2015]. [Crossref] [PubMed]
- Orandi BJ, Alachkar N, Kraus ES, et al. Presentation and outcomes of C4d-ngative antibody-mediated rejection after kidney transplantation. Am J Transplant 2016;16:213-20. [Crossref] [PubMed]
- Burke CM, Glanville AR, Theodore J, et al. Lung immunogenicity, rejection, and Obliterative Bronchiolitis. Chest 1987;92:547-9. [Crossref] [PubMed]
- Griffith BP, Paradis IL, Zeevi A, et al. Immunologically mediated disease of the airways after pulmonary transplantation. Ann Surg 1988;208:371-8. [Crossref] [PubMed]
- Cooper JD, Billingham M, Egan T, et al. A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allografts. International Society for Heart and Lung Transplantation. J Heart Lung Transplant 1993;12:713-6. [PubMed]
- Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant 2002;21:297-310. [Crossref] [PubMed]
- Pakhale SS, Hadjilliadis D, Howell DN, et al. Upper lobe fibrosis: a novel manifestation of chronic allograft dysfunction in lung transplantation. J Heart Lung Transplant 2005;24:1260-8. [Crossref] [PubMed]
- Woodrow JP, Shlobin OA, Barnett SD, et al. Comparison of bronchiolitis obliterans syndrome to other forms of chronic lung allograft dysfunction after lung transplantation. J Heart Lung Transplant 2010;29:1159-64. [Crossref] [PubMed]
- Sato M, Waddell TK, Wagnetz U, et al. Restrictive Allograft Syndrome (RAS): A novel form of chronic lung allograft dysfunction. J Heart Lung Transplant 2011;30:735-42. [Crossref] [PubMed]
- Sato M, Hwang DM, Waddell TK, et al. Progression pattern of restrictive allograft syndrome after lung transplantation. J Heart Lung Transplant 2013;32:23-30. [Crossref] [PubMed]
- Ofek E, Sato M, Saito T, et al. Restrictive allograft syndrome post lung transplantation is characterized by pleuroparenchymal fibroelastosis. Mod Pathol 2013;26:350-6. [Crossref] [PubMed]
- Burke CM, Theodore J, Dawkins KD, et al. Post-transplant obliterative bronchiolitis and other late lung sequelae in human heart-lung transplantation. Chest 1984;86:824-9. [Crossref] [PubMed]
- Yousem SA, Burke CM, Billingham ME. Pathologic pulmonary alterations in long-term human heart-lung transplantation. Hum Pathol 1985;16:911-23. [Crossref] [PubMed]
- Choi YW, Rossi SE, Palmer SM, et al. Bronchiolitis obliterans syndrome in lung transplant recipients: correlation of computed tomography findings with bronchiolitis obliterans syndrome stage. J Thorac Imaging 2003;18:72-9. [Crossref] [PubMed]
- Corris PA, Ryan VA, Small T, et al. A randomised controlled trial of azithromycin therapy in bronchiolitis obliterans syndrome (BOS) post lung transplantation. Thorax 2015;70:442-50. [Crossref] [PubMed]
- Snell GI, Esmore DS, William TJ. Cytolytic therapy for the bronchiolitis obliterans syndrome complicating lung transplantation. Chest 1996;109:874-8. [Crossref] [PubMed]
- Date H, Lynch JP, Sundaresan S, et al. The impact of cytolytic therapy on bronchiolitis obliterans syndrome. J Heart Lung Transplant 1998;17:869-75. [PubMed]
- Reams BD, Musselwhite LW, Zaas DW, et al. Alemtuzumab in the treatment of refractory acute rejection and bronchiolitis obliterans syndrome after human lung transplantation. Am J Transplant 2007;7:2802-8. [Crossref] [PubMed]
- Morrell MR, Despotis GJ, Lublin DM, et al. The efficacy of photopheresis for bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant 2010;29:424-31. [Crossref] [PubMed]
- Jaksch P, Scheed A, Keplinger M, et al. A prospective interventional study on the use of extracorporeal photopheresis in patients with bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant 2012;31:950-7. [Crossref] [PubMed]
- Greer M, Dierich M, De Wall C, et al. Phenotyping established chronic lung allograft dysfunction predicts extracorporeal photopheresis response in lung transplant patients. Am J Transplant 2013;13:911-8. [Crossref] [PubMed]
- Finlen Copeland CA, Snyder LD, Zaas DW, et al. Survival after bronchiolitis obliterans syndrome among bilateral lung transplant recipients. Am J Respir Crit Care Med 2010;182:784-9. [Crossref] [PubMed]
- Palmer SM, Limaye AP, Banks M, et al. Extended valganciclovir prophylaxis to prevent cytomegalovirus after lung transplantation: a randomized, controlled trial. Ann Intern Med 2010;152:761-9. [Crossref] [PubMed]
- Shields RK, Clancy CJ, Minces LR, et al. Staphylococcus aureus infections in the early period after lung transplantation: epidemiology, risk factors, and outcomes. J Heart Lung Transplant 2012;31:1199-206. [Crossref] [PubMed]
- Khalifah AP, Hachem RR, Chakinala MM, et al. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med 2004;170:181-7. [Crossref] [PubMed]
- Gregson AL, Wang X, Injean P, et al. Staphylococcus via an interaction with the ELR+ CXC chemokine ENA-78 is associated with BOS. Am J Transplant 2015;15:792-9. [Crossref] [PubMed]
- Gottlieb J, Mattner F, Weissbrodt H, et al. Impact of graft colonization with gram-negative bacteria after lung transplantation on the development of bronchiolitis obliterans syndrome in recipients with cystic fibrosis. Respir Med 2009;103:743-9. [Crossref] [PubMed]
- Vos R, Vanaudenaerde BM, Geudens N, et al. Pseudomonal airway colonisation: risk factor for bronchiolitis obliterans syndrome after lung transplantation? Eur Respir J 2008;31:1037-45. [Crossref] [PubMed]
- Weigt SS, Elashoff RM, Huang C, et al. Aspergillus colonization of the lung allograft is a risk factor for bronchiolitis obliterans syndrome. Am J Transplant 2009;9:1903-11. [Crossref] [PubMed]
- Weigt SS, Copland CA, Derhovanessian A, et al. Colonization with small conidia Aspergillus species is associated with bronchiolitis obliterans syndrome: a two-center validation study. Am J Transplant 2013;13:919-27. [Crossref] [PubMed]
- Botha P, Archer L, Anderson RL, et al. Pseudomonas aeruginosa colonization of the allograft after lung transplantation and the risk of bronchiolitis obliterans syndrome. Transplantation 2008;85:771-4. [Crossref] [PubMed]
- Belperio J, Palmer SM, Weigt SS. Host-pathogen interactions and chronic lung allograft dysfunction. Ann Am Thorac Soc 2017;14:S242-6. [Crossref] [PubMed]


