
Ex vivo lung perfusion prior to transplantation: an overview of current clinical practice worldwide
Introduction
Since its first description in 1963 (1), lung transplantation is now accepted as a life-saving treatment in numerous forms of end-stage lung disease. But since the beginning of the experience, organ shortage has always been an issue. The large gap between patients waiting for transplant and the number of available lungs is responsible for a mortality rate on the waiting list of up to 30% (2).
Acceptance of extended-criteria donors, donation after circulatory death (DCD) donors, and single-lung and lobar lung transplantation have been the first responses to address the problem of organ shortage. Recently, a new solution has emerged in an effort to augment the number of acceptable lungs: ex vivo lung perfusion (EVLP). After procurement and cold flush, lungs are cannulated on an isolated circuit while perfused and ventilated at normothermia for several hours prior to transplantation. Compared to cold storage (CS) as the gold standard for lung preservation nowadays, EVLP permits continued evaluation, transportation, and reconditioning of the organ. The decision to transplant the lungs may be delayed until after final multidisciplinary evaluation. The lung transplant community is hopeful that EVLP in the future offers a platform to even repair lungs by immunomodulation or gene therapy, as a technique to prevent ischemia-reperfusion injury and primary graft dysfunction (PGD) (3).
In this article, we will focus on the worldwide experience with EVLP. We systematically reviewed all series reported in literature and aimed to compare these papers with regards to the technique and protocol used, inclusion and exclusion criteria of donors and recipients, lung acceptance rate after EVLP and reported outcome after transplantation.
General considerations
EVLP is a relatively new technique. The first center utilizing this new technology was the group of Stig Steen in Lund, Sweden in 2006 (4). The group of Toronto has thereafter largely contributed to the spreading of the technique worldwide, publishing their own technique and the first reports on its large-scale utilization (5-7). After extensive research, we identified 30 publications from 24 centers in 14 countries: UK, Canada, Australia, USA, Spain, Belgium, Brazil, Denmark, Germany, France, Sweden, Iran, Italy and Austria. This represent about 550 donors in whom the lungs were evaluated on an EVLP device. Papers from Iran (8) and Brazil (9,10), however, only described experience with lung cannulation and reconditioning on the EVLP circuit, but no transplantation followed. Their technique and results therefore will not be discussed in the present review.
Only two papers reported on a randomized control trial: INSPIRE trial (11) and VIENNA trial (12). The other 26 papers were retrospective controlled or prospective single-arm studies. The outcome of three others trials are still awaited (NOVEL, EXPAND and PERFUSIX trial) with interim results already presented at international meetings (13).
Technique
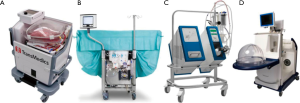
Four commercial systems are available on the market nowadays (Figure 1): OCSTM Lung (Transmedics, Andover, USA), Lung AssistTM (Organ Assist, Groningen, The Netherlands), XPSTM and LSTM (XVIVO, Göteborg, Sweden). In addition to these devices, some centers are using their own home-made system. Clinical experience with transplantation after EVLP using these devices has been published so far, even for the Lung AssistTM recently (15). Details of the technical aspects and functioning of all EVLP devices is beyond the scope of this review (Table 1). We would like to focus on some critical steps.
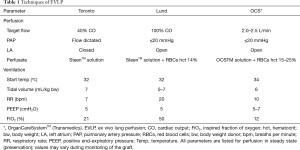
Full table
Basically, there are three EVLP protocols currently used worldwide: Toronto, Lund, and Organ Care SystemTM (OCS, Transmedics, Andover, MA, USA). These protocols differ by the perfusate used, target flow, pulmonary arterial pressure, left atrial pressure, and ventilatory settings.
Interestingly, some centers have reported a modification to these protocols. For example, in the DEVELOP-UK trial (16), the technique used for the first 22 donor lungs was a hybrid Toronto/Lund technique. The left atrium was left open, the perfusate was acellular and the flow was limited to 40% to 60% of donor cardiac output. After preliminary results in the first 22 EVLP patients and given the high number of extracorporeal support needed after transplantation, the investigators decided to switch entirely to the original Lund technique. At that time, the experience worldwide with the Vivoline device and the Lund protocol was growing and the hope was great that this would boost the conversion rate of lungs transplanted after EVLP reported to be >80% in other series.
The Gothenburg group (17-19) used the Lund technique, but included lots of minor differences (such as use of more careful ventilation and perfusion parameters during the reconditioning phase) derived from their own experiments.
In total we counted 5 centers using the Toronto technique, 6 using the Lund technique, and 24 using OCS method. As already stated, some centers modified the originally described procedure to their own experience. Importantly, the vast majority of these reports didn’t provide enough details to perform a real comparison between the procedures (Table 2).
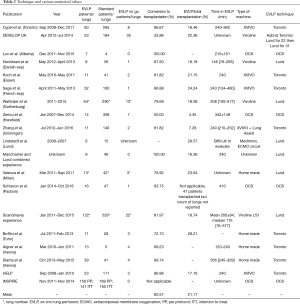
Full table
Criteria for transplantation after EVLP (Table 3)
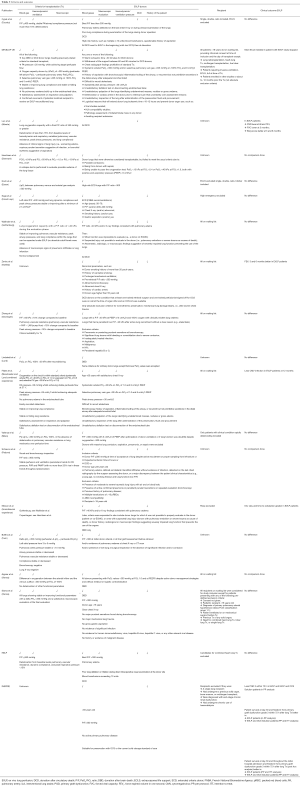
Full table
Most teams use the following combination of acceptance criteria after reconditioning to decide if the lungs are suitable for transplantation:
- Gas exchange at end of evaluation phase: several strategies are reported.
- PaO2/FiO2 >350 mmHg with PaO2 being measured in blood sample from the left atrium. This cut-off value varies between teams ranging from 300 to 400 mmHg. There is currently no universally accepted threshold;
- Delta left atrium PaO2 − pulmonary artery PaO2 >350 mmHg;
- (Perfusate left atrium PaO2 − perfusate pulmonary artery PaO2)/FiO2 >300 mmHg;
- PCO2 <6 kPa (45.6 mmHg) and PO2 >50 kPa (380 mmHg) at FiO2 =1.0 or PO2 >13 kPa (98.8 mmHg) at FiO2 =0.21 as reported by the Danish team (20). These blood gas values are recorded after deoxygenation of the perfusate by the gas exchanger in the circuit.
These criteria may be used together as reported in the DEVELOP-UK and Manchester-Lund reports (21), where the authors used a combination of the arterial blood gas/ratio and selective pulmonary vein gas.
- Hemodynamic and ventilatory parameters: pulmonary artery and peak airway pressure, lung compliance and lung resistance. For most of the centers these parameters have to remain stable. However, a certain degree of deterioration is often permitted but a strict value or threshold for declining the organ for transplantation was never used. The group at the University of Alberta (22,23), the investigators of the HELP trial (5) and the Expand trial reported a threshold of maximum 15% of deterioration of these parameters as an acceptable criterion for transplantation.
- Macroscopic evaluation of the lungs: absence of oedema at palpation or bronchoscopy, purulent secretion, erythema of the bronchus (suggestive of aspiration), negative X-ray and satisfactory lung deflation after endotracheal tube disconnection (collapse test). Again, absolutely no strict guidelines were found. The utilization of these criteria by the different teams that reported their experience is erratic.
The mean number of transplanted lungs after EVLP reported in the series was 80.57%. The acceptance rate varies from one center to another from 34% (DEVELOP-UK) to 97% in the French experience (24). However, many centers transplant more than 80% of the lungs reconditioned on an EVLP device (Table 2).
From several reports that used EVLP with otherwise rejected lungs [Paris, Essen (25), Gothenburg and Copenhagen (26), Groningen, Lund (27), Milan (28,29), Turin (30) and Vienna 2012 (31)] we can also conclude that EVLP is a good tool to increase the number of lung transplants, with a mean increase of 21.17% (range, 7.28–28.57%). This brings hope that with greater acceptance of EVLP, mortality rate on waiting list will probably fall in the coming years. For example, the Foch Center in Paris reported a mean waiting time for patients of 3 weeks, which represent a dramatic decrease since the beginning of their EVLP program (Table 2).
EVLP donors (Table 3)
Two categories of donors were reported.
The vast majority of the studies that we found used an EVLP device for reconditioning of extended-criteria lungs or lungs that otherwise would have been rejected for transplantation. Needless to say, every team reporting their individual experience had different criteria to decide whether or not a lung is deemed suitable for transplantation. These criteria can be summarized as follows:
- Blood gases: best PaO2/FiO2 <300 mmHg, systemic arterial PaO2 <300 mmHg, pulmonary vein gas <225 mmHg;
- Macroscopic evaluation: absence of oedema, contusion, atelectasis difficult to recruit, mass/nodules/edema on palpation, poor lung compliance, abnormal bronchoscopy, abnormal chest X-ray;
- Lungs from DCD donors: this criterion is used by some teams (e.g., Alberta group) as an absolute criterion to evaluate lungs on EVLP prior to transplantation. Other centers (e.g., Toronto group) used EVLP for DCD lungs at the start of their experience at the surgeon’s discretion;
- Donor profile: multiple transfusion, history of aspiration, extended ischemic time, sepsis, age, smoking history, severe trauma, pulmonary embolism, prolonged mechanical ventilation, history of cardiac arrest.
These criteria are usually combined, but may also be used alone (especially for the DCD criterion and the blood gases).
Some teams also reported a modification of their use of EVLP over time. For example, in the Scandinavian experience, at the beginning, the authors only used blood gases and X-ray findings to accept EVLP lungs. Later, the criteria were expanded to also include patients bridged to lung transplantation on extracorporeal life support (ECLS), injured lungs and severely impaired lungs on macroscopic and radiological evaluation. In the Toronto experience, all DCD donors’ lungs were included for EVLP then at the surgeon’s discretion.
Two studies evaluated the use of EVLP for normothermic preservation of standard-criteria lungs compared to CS (Vienna 2017 and INSPIRE trial).
Donor inclusion criteria used were:
- Blood samples: best PaO2/FiO2 >300 mmHg, no viral infection (HIV, hepatitis B/C, …);
- Macroscopic evaluation: clear chest X-ray, normal bronchoscopy, no evidence of lung infection/malignant disease;/
- Donor age (>18 and <65 years);
- No history of aspiration, neither trauma.
Exclusion criteria to prevent useless EVLP therapy were:
- Mechanical lung damage (tears) leading to air/blood leaks;
- Massive lung contusion;
- Pneumonia;
- Sepsis or aspiration;
- Multiple RBC transfusion;
- Recipient <18 years;
- ABO incompatibility.
The description of the EVLP donors is generally well reported by the teams worldwide.
EVLP recipients (Table 3)
Patients who may benefit from a lung transplantation after reconditioning with EVLP are poorly described and usually not discussed in the different reports we examined.
The Toronto and Essen group included patients receiving single, double or even redo lung transplantation. Only patients under ECLS and patients requiring heart-lung transplantation were excluded.
In DEVELOP-UK, EVLP was reserved for adult patients >18 years requiring no lung/heart assistance and no redo/multiorgan/lobar/living donor lung transplantation.
The Paris team excluded high emergency patients while the Milan team (25,26) choose to include only patients with rapidly deteriorating clinical status.
Only Gothenburg and Harefield (32,33) teams included all patients on waiting list.
Concerning the studies evaluating standard lungs with EVLP, recipients were excluded if they were pediatric patients (<18 years), suffered from pulmonary arterial hypertension, need for heart/lung assisting device prior to transplantation (VIENNA 2017) or if they presented with severe renal dysfunction (INSPIRE). Both studies included double-lung transplantation only and excluded patients with previous transplant.
EVLP outcomes (Table 3)
In most of the reports, outcome was compared with a control group showing no major difference between reconditioned and standard lungs except in the experience reported from Denmark, Padova (34) and Vienna 2012. In many reports total cross clamp time (including cold and warm ischemic times) in EVLP transplants was greater compared to cold stored lungs. Lungs on EVLP are however fed with nutrients and constantly ventilated. Therefore, these lungs are not exposed to the risk of ischemic damage while stored outside the body.
Clinically, the Alberta team (evaluating EVLP only for DCD lungs) demonstrated a significantly lower rate of PGD at 72 h after transplant for the lungs undergoing EVLP compared to standard lungs (0.4±0.5 vs. 2.1±0.7, P=0.003). Three months after lung transplantation, FVC% and Borg Dyspnea Score was worse in patients transplanted with EVLP lungs. The authors stated that the explanation for such results is unknown and should be validated in a larger prospective study. In the Scandinavian experience, the patients treated with EVLP lungs were found to have longer ICU stay and time to extubation compared to recipients of standard lungs. The explanation could be that the EVLP lungs are in worse condition than the standard lungs and that the time from retrieval to transplantation was also longer.
Manchester and Lund experience reported that patients with EVLP lungs were more prone to symptomatic CMV infection 90 days from the transplantation. However, all patients developing a CMV infection in these studies were high/intermediate risk patients from a serologic point of view. This probably explains these negative findings better than the EVLP process itself.
Conclusions
EVLP is certainly a useful technique for the future. We have seen that, in numerous centers, this tool permits to really expand the number of lungs available for transplantation and to decrease the waiting time for patients. The clinical outcomes of EVLP treated lungs are as good as favorable lungs in terms of PGD and overall survival and the new perspective of treating lungs while on EVLP is a promising research field for the future.
However, there is a need to standardize the procedure. The techniques, the criteria for recipient and donor lung selection and the duration of EVLP are very different from one study to another making them difficult to reproduce. Several study reports are thus difficult to compare because of the potential bias in the inclusion criteria. To our knowledge this review represents the most complete overview of the worldwide utilization of EVLP nowadays.
Acknowledgements
None.
Footnote
Conflicts of Interest: D Van Raemdonck is a member of the scientific advisory board of Transmedics. The other authors have no conflicts of interest to declare.
References
- Hardy JD, Webb WR, Dalton ML Jr, et al. Lung homotransplantation in man JAMA 1963;186:1065-74. [Crossref] [PubMed]
- Gottlieb J. Lung allocation. J Thorac Dis 2017;9:2670-4. [Crossref] [PubMed]
- Van Raemdonck D, Rega F, Rex S, et al. Machine perfusion of thoracic organs. J Thorac Dis 2018;10:S910-23. [Crossref] [PubMed]
- Ingemansson R, Eyjolfsson A, Mared L, et al. Clinical transplantation of initially rejected donor lungs after reconditioning ex vivo. Ann Thorac Surg 2009;87:255-60. [Crossref] [PubMed]
- Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med 2011;364:1431-40. [Crossref] [PubMed]
- Cypel M, Yeung JC, Hirayama S, et al. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant 2008;27:1319-25. [Crossref] [PubMed]
- Cypel M, Yeung JC, Machuca T, et al. Experience with the first 50 ex vivo lung perfusions in clinical transplantation. J Thorac Cardiovasc Surg 2012;144:1200-6. [Crossref] [PubMed]
- Shafaghi S, Najafizadeh K, Sheikhy K, et al. The First Experience of Ex-Vivo Lung Perfusion (EVLP) in Iran: An Effective Method to Increase Suitable Lung for Transplantation. Int J Organ Transplant Med 2016;7:219-27. [PubMed]
- Abdalla LG, Braga KA, Nepomuceno NA, et al. Ex vivo lung perfusion in Brazil. J Bras Pneumol 2016;42:95-8. [Crossref] [PubMed]
- Pêgo-Fernandes PM, de Medeiros IL, Mariani AW, et al. Ex vivo lung perfusion: early report of Brazilian experience. Transplant Proc 2010;42:440-3. [Crossref] [PubMed]
- Warnecke G, Van Raemdonck D, Smith MA, et al. Normothermic ex-vivo preservation with the portable Organ Care System Lung device for bilateral lung transplantation (INSPIRE): a randomised, open-label, non-inferiority, phase 3 study. Lancet Respir Med 2018;6:357-67. [Crossref] [PubMed]
- Slama A, Schillab L, Barta M, et al. Standard donor lung procurement with normothermic ex vivo lung perfusion: A prospective randomized clinical trial. J Heart Lung Transplant 2017;36:744-753. [Crossref] [PubMed]
- Van Raemdonck D, Neyrinck A, Cypel M, et al. Ex-vivo lung perfusion. Transpl Int 2015;28:643-56. [Crossref] [PubMed]
- Van Raemdonck D, Neyrinck A, Rega F, et al. Machine perfusion in organ transplantation: a tool for ex-vivo graft conditioning with mesenchymal stem cells? Curr Opin Organ Transplant 2013;18:24-33. [Crossref] [PubMed]
- Zhang ZL, van Suylen V, van Zanden JE, et al. First experience with ex vivo lung perfusion for initially discarded donor lungs in the Netherlands: a single-centre study. Eur J Cardiothorac Surg 2018. [Epub ahead of print]. [PubMed]
- Fisher A, Andreasson A, Chrysos A, et al. An observational study of Donor Ex Vivo Lung Perfusion in UK lung transplantation: DEVELOP-UK. Health Technol Assess 2016;20:1-276. [Crossref] [PubMed]
- Wallinder A, Riise GC, Ricksten SE, et al. Transplantation after ex vivo lung perfusion: A midterm follow-up. J Heart Lung Transplant 2016;35:1303-10. [Crossref] [PubMed]
- Wallinder A, Ricksten SE, Silverborn M, et al. Early results in transplantation of initially rejected donor lungs after ex vivo lung perfusion: a case-control study. Eur J Cardiothorac Surg 2014;45:40-4; discussion 44-5. [Crossref] [PubMed]
- Wallinder A, Ricksten SE, Hansson C, et al. Transplantation of initially rejected donor lungs after ex vivo lung perfusion. J Thorac Cardiovasc Surg 2012;144:1222-8. [Crossref] [PubMed]
- Henriksen IS, Møller-Sørensen H, Møller CH, et al. First Danish experience with ex vivo lung perfusion of donor lungs before transplantation. Dan Med J 2014;61:A4809. [PubMed]
- Fildes JE, Archer LD, Blaikley J, et al. Clinical Outcome of Patients Transplanted with Marginal Donor Lungs via Ex Vivo Lung Perfusion Compared to Standard Lung Transplantation. Transplantation 2015;99:1078-83. [Crossref] [PubMed]
- Bozso S, Vasanthan V, Luc JG, et al. Lung transplantation from donors after circulatory death using portable ex vivo lung perfusion. Can Respir J 2015;22:47-51. [Crossref] [PubMed]
- Luc JGY, Jackson K, Weinkauf JG, et al. Feasibility of Lung Transplantation from Donation After Circulatory Death Donors Following Portable Ex Vivo Lung Perfusion: A Pilot Study. Transplant Proc 2017;49:1885-92. [Crossref] [PubMed]
- Sage E, Mussot S, Trebbia G, et al. Lung transplantation from initially rejected donors after ex vivo lung reconditioning: the French experience. Eur J Cardiothorac Surg 2014;46:794-9. [Crossref] [PubMed]
- Koch A, Pizanis N, Olbertz C, et al. One-year experience with ex vivo lung perfusion: Preliminary results from a single center. Int J Artif Organs 2018;41:460-6. [Crossref] [PubMed]
- Nilsson T, Wallinder A, Henriksen I, et al. Lung transplantation after ex vivo lung perfusion in two Scandinavian centres. Eur J Cardiothorac Surg 2019;55:766-72. [Crossref] [PubMed]
- Lindstedt S, Hlebowicz J, Koul B, et al. Comparative outcome of double lung transplantation using conventional donor lungs and non-acceptable donor lungs reconditioned ex vivo. Interact Cardiovasc Thorac Surg 2011;12:162-5. [Crossref] [PubMed]
- Valenza F, Citerio G, Palleschi A, et al. Successful Transplantation of Lungs From an Uncontrolled Donor After Circulatory Death Preserved In Situ by Alveolar Recruitment Maneuvers and Assessed by Ex Vivo Lung Perfusion. Am J Transplant 2016;16:1312-8. [Crossref] [PubMed]
- Valenza F, Rosso L, Gatti S, et al. Extracorporeal lung perfusion and ventilation to improve donor lung function and increase the number of organs available for transplantation. Transplant Proc 2012;44:1826-9. [Crossref] [PubMed]
- Boffini M, Ricci D, Bonato R, et al. Incidence and severity of primary graft dysfunction after lung transplantation using rejected grafts reconditioned with ex vivo lung perfusion. Eur J Cardiothorac Surg 2014;46:789-93. [Crossref] [PubMed]
- Aigner C, Slama A, Hötzenecker K, et al. Clinical ex vivo lung perfusion--pushing the limits. Am J Transplant 2012;12:1839-47. [Crossref] [PubMed]
- Zeriouh M, Sabashnikov A, Mohite PN, et al. Utilization of the organ care system for bilateral lung transplantation: preliminary results of a comparative study. Interact Cardiovasc Thorac Surg 2016;23:351-7. [Crossref] [PubMed]
- Zych B, Popov AF, Stavri G, et al. Early outcomes of bilateral sequential single lung transplantation after ex-vivo lung evaluation and reconditioning. J Heart Lung Transplant 2012;31:274-81. [Crossref] [PubMed]
- Schiavon M, Faggi G, Rebusso A, et al. Extended criteria donor lung reconditioning with the organ care system lung: a single institution experience. Transpl Int 2019;32:131-40. [Crossref] [PubMed]


