
Trophinin-associated protein expression is an independent prognostic biomarker in lung adenocarcinoma
Introduction
According to the latest reports from A Cancer Journal for Clinicians (CA), lung cancer remains the leading cause of cancer incidence and cancer-related mortality worldwide (1), while adenocarcinoma represents the most common histological subtype (2). Despite recent advances in various treatment options during the last decade (including surgery, chemotherapy, targeted therapy, immunotherapy), the 5-year survival of LAC has not been improved to a satisfactory standard (3), and TNM staging remains the most important prognostic factor for predicting the recurrence rates and survival times of LAC patients (4). In addition to these classical clinical methods, novel prognostic tools based on individual tumor mutations and protein expression, such as WD repeat domain 62 (WDR62) (5), lncRNA forebrain embryonic zinc finger protein 1 antisense RNA1 (FEZF1-AS1) (6), and transmembrane protease serine 4 (TMPRSS4) (7), have shown great potential in prognostic prediction. However, none of these genes have been generally acknowledged in clinical practice, which means a more stable, convenient and reliable prognostic biomarker is needed for LAC.
Trophinin-associated protein (TROAP), formally known as tastin, was first identified as a cytoplasmic protein that mediated the initial attachment of the trophoblast to the endometrial epithelium in early embryo implantation by forming a complex with trophinin and bystin (8-11). Previous studies showed that TROAP mRNA have higher expression levels in testis, bone marrow, and thymus (9,12). Additionally, the functions of the trophinin-tastin-bystin complex seemed to be only associated with embryo implantation. However, recent studies revealed that TROAP could regulate proper spindle assembly during mitosis as microtubule-associated proteins, while the loss of TROAP expression led to mitotic block and multipolar spindles (13).
In terms of malignant cancers, since TROAP was found highly expressed in human cancer cell lines (HeLa and Jurkat cells) (13,14), more and more studies have been conducted to investigate the relationships between TROAP and various cancers. Until now, high expression of TROAP was reported to be related to poorer outcomes in ovarian epithelial carcinoma (12,15), gastric cancer (16), colorectal cancer (17), and hepatocellular carcinoma (18). As a novel prognostic predictor, the relationship between TROAP expression and other types of cancers, including LAC, remains unclear. Given this situation, we evaluated the potential correlations between TROAP expression in LAC tissues and clinical pathologic information of these patients by analyzing the data from the TCGA lung cancer database and the UCSC Xena database. Furthermore, we assessed whether TROAP expression could be an independent prognostic biomarker for the overall survival of LAC patients.
Methods
Data collecting
The RNA-sequencing (RNA-Seq) expression data (level 3 data) was obtained from the TCGA lung cancer database (http://cancergenome.nih.gov/) with the RTCGAToolbox package in R (19), and the full clinical pathologic information of the corresponding patients was downloaded from the UCSC Xena database (https://xenabrowser.net/datapages/) according to their ID in TCGA. The expression value of TROAP mRNA was converted to normalized RNA-Seq by Expectation-Maximization (RSEM) values for further statistical analysis. The detailed pre-processing steps, including mapping and normalization, are described on the UCSC Xena website (http://xena.ucsc.edu/).
Statistical analysis
To evaluate the relationships between TROAP expression and clinical, pathologic parameters, high and low TROAP expression groups were defined by the median value of the TROAP expression. The correlation between TROAP expression and clinical, pathologic parameters was tested by the Chi-square tests using SPSS software version 19.0. Differences in overall survival between high and low expression groups were compared using Kaplan-Meier curves, and P values were calculated by a log-rank test. The survival package in R was used for the above tests. Univariate Cox regression analysis of the TROAP expression, along with other clinical, pathologic parameters, was used to estimate survival rates and screen the possible variables that may affect overall survival. Multivariate Cox analysis was performed to evaluate the effects of TROAP expression on overall survival, along with other clinical parameters which were correlated with overall survival in univariate analysis (T, N, and M stage, clinical stage, and radiation therapy history). This study was approved by the Medical Ethics Committee of the Sir Run Run Shaw Hospital affiliated to Zhejiang University School of Medicine (No. 20190319-18).
Results
Patient characteristics
From TCGA lung cancer database, the RNA-Seq expression data of 594 patients were collected, among which data from 66 patients were excluded due to patient TCGA ID being unable to be located on the UCSC Xena database. The final 528 samples with both TROAP expression information and full clinical characteristics were then submitted for statistical analysis. The demographic and clinical pathologic characteristics, including TNM stage, the location of the tumor, survival status, radiation therapy and smoking history, of the corresponding patients are described in Table 1.
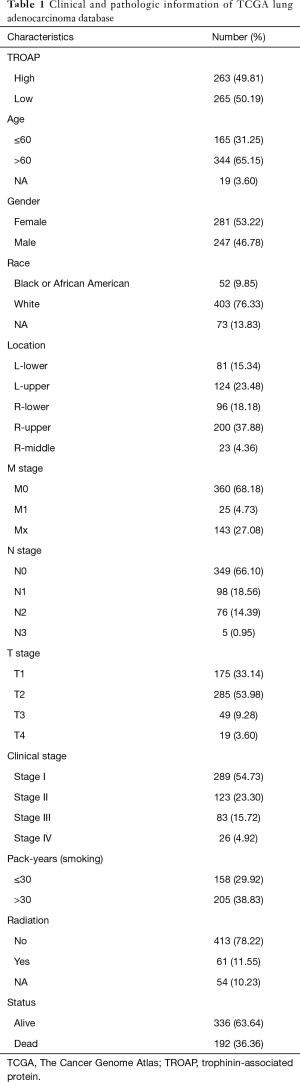
Full table
TROAP expression was associated with clinical pathologic parameters of LAC
The necessary information of patients, including TROAP expression, age, gender, race, smoking history, TNM stage, and clinical stage, as well as the use of radiation therapy, and survival status are shown in Table 1. In order to evaluate the relationship between TROAP expression and clinical, pathologic parameters of LAC further, patients were divided into high and low TROAP expression groups according to the median value of TROAP expression. According to the Chi-square tests, we found high TROAP expression correlated with younger age (≤60) (P=0.047), male (P=0.005), earlier stage of T stage (P=0.011), N stage (P=0.017), M stage (P=0.022), and TNM (P=0.007), and longer smoking history (>30 pack-year) (P<0.001) (Table 2).
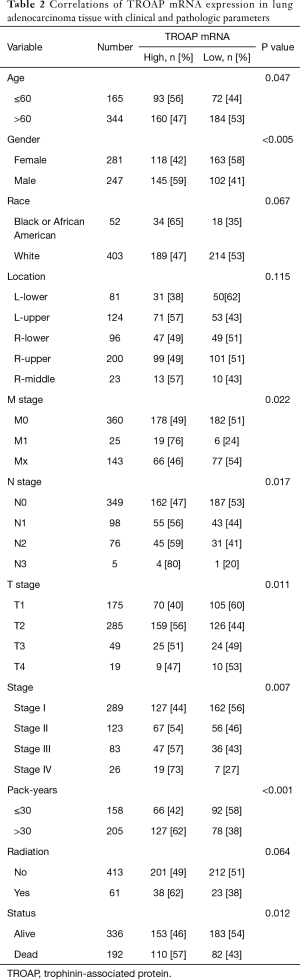
Full table
TROAP was an independent prognostic factor for poor overall survival in LAC
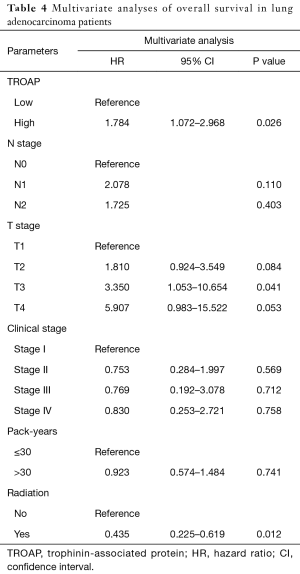
The further subgroup analysis on the Kaplan-Meier curves of overall survival and log-rank tests showed that high TROAP expression might be associated with poor overall survival of patients in the T3 stage (P=0.0013), N0 stage (P=0.014), and M0 stage (P=0.0023) (Figure S1). Furthermore, during univariate analysis, TROAP expression, T stage, N stage, clinical stage, and radiation therapy history were correlated with poor overall survival (Table 3). The following multivariate analysis confirmed that high TROAP expression was an independent prognostic factor for poor overall survival of LAC patients [hazard ratio (HR): 1.784, 95% confidence interval (CI): 1.072–2.968, P=0.026; Table 4].
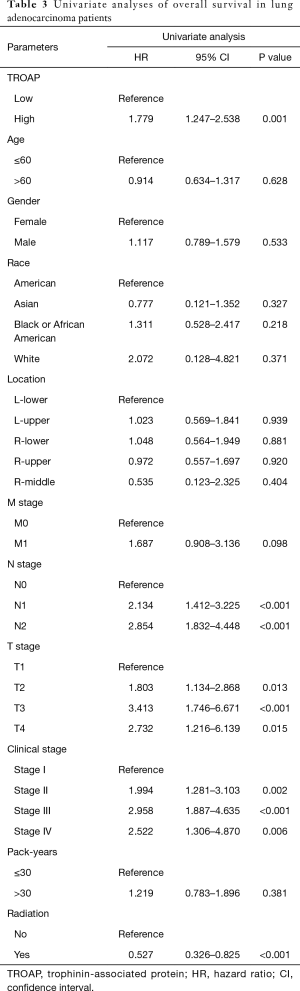
Full table
Full table
Discussion
The present study demonstrated that high TROAP expression was correlated with younger age (≤60), male sex, earlier stage of T, N, M and TNM, and a longer smoking history (>30 pack-year). Patients with higher TROAP expressions in LAC tissues related to a poorer prognosis generally, especially those in T3 stage, N0 stage and M0 stage. Moreover, TROAP expression could be a reliable independent prognostic biomarker for LAC patients in clinical practice.
Consistent with all previous studies (12,15-18), we observed higher TROAP expression levels in LAC tumor tissues with data mining from the TCGA lung cancer database. Conversely, Lian et al. reported decreased mRNA and protein expression of TROAP in Chinese hepatocellular carcinoma tissues, most of which were originated from hepatitis B virus -infected patients (20). This discrepancy was attributed to the different origins of the detection samples. An opposite result was presented by Yan et al. from a study based on the TCGA Liver Hepatocellular Carcinoma (TCGA-LHC) data. In Yan’s study, all hepatocellular carcinoma samples caused by alcohol, hepatitis B virus, hepatitis C virus, and nonalcoholic steatohepatitis were included (18). This discrepancy reminded us that molecular pathogenesis could be different in the same type of cancer according to their etiology. Moreover, based on a better understanding of the mechanisms of tumorigenesis, we suggest new molecular classifications of various cancers, including lung cancer, should be identified despite existing clinical and pathologic classifications. These molecular classifications, with the potential to revolutionize present treatment principles, could be reliable and accurate prognostic factors for corresponding cancers.
The spindle assembly checkpoint, ensuring the fidelity of chromosome segregation to produce genetically identical daughter cells, is the major cell cycle control mechanism in mitosis and is essential to reducing genomic instability during cell cycle progression (21). Spindle assembly and function are intimately associated with microtubule dynamics spatially and temporally during the cell cycle (22). Along with other centrosomal and noncentrosomal proteins (23), TROAP helps to maintain the structural and dynamic features of centrosomes and contributes to normal spindle functioning as a microtubule-associated protein (24). During mitosis, two critical events, bipolar spindle assembly and centrosome integrity were controlled by TROAP, which was supposed to be essential for the microtubular cytoskeleton (8,14). A previous study showed that TROAP overexpression was associated with tumorigenesis and clinical pathologic characteristics of breast cancer, such as advanced stage, rapid speed of mitosis, and enhanced expression of several oncogenes (HER2, TOP2A and EGFR), while TROAP was hardly expressed in normal breast tissues (25). Li et al. found that the methylation of TROAP, one of the top 5 most significant phase-specific genes in HeLa and embryonic stem cells, would execute numerous functions to promote carcinogenesis in the G2 phase (26). In this study, we found that TROAP expression was strongly associated with T stage, N stage, M stage, and clinical stage in LAC. Thus we supposed that TROAP could promote cellular proliferation and tumor growth in LAC by propelling cell cycle progression.
Fukuda and Sugihara reported that the cell adhesion molecules, including L-selectin and trophinin, play a pivotal role in human embryo implantation (11). Trophinin is a membrane protein which is supposed to have self-binding activity and thus mediates homophilic cell adhesion, while TROAP is a cytoplasmic protein required for trophinin to exhibit cell adhesion activity (9). In trophoblastic cells, once trophinin binds to TROAP in the cytoplasm, the extracellular domain of trophinin can function as a cell adhesion molecule (8). After the initial attachment of embryonic cells to the maternal epithelial cells, a stronger adhesion is induced, and significant morphological changes are observed in the embryo implantation site. Aggressive behaviors of trophoblasts during embryo implantation resemble those of malignant tumor cells, and it is not surprising if some mechanisms are shared by trophoblasts and cancer cells (10,27). Indeed, Chen et al. identified trophinin as an enhancer for cell invasion and a prognostic factor for early-stage lung cancer (28). Moreover, another previous study has confirmed that knockdown of TROAP, targeted by miR-519d-3p, significantly suppressed cell proliferation, migration and invasion, inducing cell cycle G0/G1 phase arrest and promoting cell apoptosis of colorectal cancer cells (17).
The present study showed that high TROAP expression was closely related to more lymph node metastasis, more distant metastasis, later clinical stages and shorter survival times. Therefore, we assume that TROAP may contribute to cancer cell proliferation, migration and invasion by regulation of microtubule-associated proteins. However, further investigations are needed to verify our hypothesis and elucidate the underlying molecular mechanisms. Multivariate analysis of this study showed that radiotherapy history was another independent prognostic factor of poor survival in LAC patients. However as we all know, treated with radiation is a clinical decision according to patient’s clinical and pathological situations, not a defining characteristic. That is to say: radiotherapy history cannot be a proper predictive factor. Inconsistent with previous studies, multivariate analysis showed that P values of T stage, N stage, M stage, and clinical stage (TNM staging) were greater than 0.05, although univariate analyses suggested that they were risk factors for survival in LAC patients. We attributed this discrepancy to a small sample size and more samples should be included to validate our results.
Although we have found an effective prognostic biomarker to predict the prognosis of LAC, the limitations of the present study should also be acknowledged. Firstly, the statistical analysis is based on the data from the TCGA database and should be validated in other cohorts and a larger number of samples in future studies. Secondly, further studies should be performed to reveal the mechanisms of TROAP involved in the cellular proliferation, migration and invasion of LAC. Finally, as some other genes and proteins reported to be related to prognosis of lung cancer patients, a proper predictive model should be generated by combining clinical, pathologic features and whole genome sequencing.
This study as far as we know is the first study to clarify the relationship between TROAP expression and clinical pathologic characteristics in LAC, and to report that TROAP may serve as an independent prognostic factor for poor survival in LAC. Such information is likely to be of great use in the management of LAC patients in the future.
Acknowledgments
Funding: This research was supported by Zhejiang Provincial Natural Science Foundation of China under Grant No. LQ19H280007.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Medical Ethics Committee of the Sir Run Run Shaw Hospital affiliated to Zhejiang University School of Medicine (No. 20190319-18).
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Guinde J, Frankel D, Perrin S, et al. Lamins in Lung Cancer: Biomarkers and Key Factors for Disease Progression through miR-9 Regulation? Cells 2018. [Crossref] [PubMed]
- Devarakonda S, Morgensztern D, Govindan R. Genomic alterations in lung adenocarcinoma. Lancet Oncol 2015;16:e342-51. [Crossref] [PubMed]
- Woodard GA, Jones KD, Jablons DM. Lung Cancer Staging and Prognosis. Cancer Treat Res 2016;170:47-75. [Crossref] [PubMed]
- Shinmura K, Kato H, Kawanishi Y, et al. WDR62 overexpression is associated with a poor prognosis in patients with lung adenocarcinoma. Mol Carcinog 2017;56:1984-91. [Crossref] [PubMed]
- Liu Z, Zhao P, Han Y, et al. LincRNA FEZF1-AS1 is associated with prognosis in lung adenocarcinoma and promotes cell proliferation, migration and invasion. Oncol Res 2018;27:39-45. [Crossref] [PubMed]
- Fan X, Liang Y, Liu Y, et al. The upregulation of TMPRSS4, partly ascribed to the downregulation of miR125a5p, promotes the growth of human lung adenocarcinoma via the NFkappaB signaling pathway. Int J Oncol 2018;53:148-58. [PubMed]
- Fukuda MN, Sato T, Nakayama J, et al. Trophinin and tastin, a novel cell adhesion molecule complex with potential involvement in embryo implantation. Genes Dev 1995;9:1199-210. [Crossref] [PubMed]
- Fukuda MN, Nozawa S. Trophinin, tastin, and bystin: a complex mediating unique attachment between trophoblastic and endometrial epithelial cells at their respective apical cell membranes. Semin Reprod Endocrinol 1999;17:229-34. [Crossref] [PubMed]
- Fukuda MN, Sugihara K, Nakayama J. Trophinin: what embryo implantation teaches us about human cancer. Cancer Biol Ther 2008;7:1165. [Crossref] [PubMed]
- Fukuda MN, Sugihara K. Cell adhesion molecules in human embryo implantation. Sheng Li Xue Bao 2012;64:247-58. [PubMed]
- Godoy H, Mhawech-Fauceglia P, Beck A, et al. Developmentally restricted differentiation antigens are targets for immunotherapy in epithelial ovarian carcinoma. Int J Gynecol Pathol 2013;32:536-40. [Crossref] [PubMed]
- Yang S, Liu X, Yin Y, et al. Tastin is required for bipolar spindle assembly and centrosome integrity during mitosis. FASEB J 2008;22:1960-72. [Crossref] [PubMed]
- Nadano D, Nakayama J, Matsuzawa S, et al. Human tastin, a proline-rich cytoplasmic protein, associates with the microtubular cytoskeleton. Biochem J 2002;364:669-77. [Crossref] [PubMed]
- Partheen K, Levan K, Osterberg L, et al. Four potential biomarkers as prognostic factors in stage III serous ovarian adenocarcinomas. Int J Cancer 2008;123:2130-7. [Crossref] [PubMed]
- Jing K, Mao Q, Ma P. Decreased expression of TROAP suppresses cellular proliferation, migration and invasion in gastric cancer. Mol Med Rep 2018;18:3020-6. [PubMed]
- Ye X, Lv H. MicroRNA-519d-3p inhibits cell proliferation and migration by targeting TROAP in colorectal cancer. Biomed Pharmacother 2018;105:879-86. [Crossref] [PubMed]
- Jiao Y, Li Y, Lu Z, et al. High Trophinin-Associated Protein Expression Is an Independent Predictor of Poor Survival in Liver Cancer. Dig Dis Sci 2019;64:137-43. [Crossref] [PubMed]
- Samur MK. RTCGAToolbox: a new tool for exporting TCGA Firehose data. PLoS One 2014;9:e106397. [Crossref] [PubMed]
- Lian Y, Fan W, Huang Y, et al. Downregulated Trophinin-Associated Protein Plays a Critical Role in Human Hepatocellular Carcinoma Through Upregulation of Tumor Cell Growth and Migration. Oncol Res 2018;26:691-701. [Crossref] [PubMed]
- Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol 2007;8:379-93. [Crossref] [PubMed]
- Srivastava S, Panda D. A centrosomal protein STARD9 promotes microtubule stability and regulates spindle microtubule dynamics. Cell Cycle 2018;17:2052-68. [Crossref] [PubMed]
- Pihan GA, Purohit A, Wallace J, et al. Centrosome defects and genetic instability in malignant tumors. Cancer Res 1998;58:3974-85. [PubMed]
- Ly TK, Wang J, Pereira R, et al. Activation of the Ran GTPase is subject to growth factor regulation and can give rise to cellular transformation. J Biol Chem 2010;285:5815-26. [Crossref] [PubMed]
- AbdelFatah T, Balls G, Miles A, et al. Abstract P6-07-09: Identification of Trophinin associated protein (TROAP) as a novel biological marker in breast cancer (BC): Co-expression of TROAP and TOPO2A predicts response of anthracycline based chemotherapy (ATC-CT). Cancer Res 2012;72:P6-07-09.
- Li CW, Chen BS. Investigating core genetic-and-epigenetic cell cycle networks for stemness and carcinogenic mechanisms, and cancer drug design using big database mining and genome-wide next-generation sequencing data. Cell Cycle 2016;15:2593-607. [Crossref] [PubMed]
- Sugihara K, Sugiyama D, Byrne J, et al. Trophoblast cell activation by trophinin ligation is implicated in human embryo implantation. Proc Natl Acad Sci U S A 2007;104:3799-804. [Crossref] [PubMed]
- Chen KY, Lee YC, Lai JM, et al. Identification of trophinin as an enhancer for cell invasion and a prognostic factor for early stage lung cancer. Eur J Cancer 2007;43:782-90. [Crossref] [PubMed]


