
Diagnostic value of ProGRP for small cell lung cancer in different stages
Introduction
Small cell lung cancer (SCLC) and large cell neuro-endocrine carcinoma (LCNEC) are aggressive neoplasms with poor prognosis (1-3). Both are generally associated with an extremely high rate of proliferation and metastasis. Therapeutic strategies for non-small cell lung cancer (NSCLC) are progressing rapidly (4-8), but there are limited cytotoxic drugs provided for SCLC and LCNEC. Thus, early and accurate detection of SCLC and LCNEC is critical. Precise detection of tumor pathology types has been pursued with routine approaches, such as the use of image-guided percutaneous transthoracic needle biopsy and bronchoscope. Despite this, those patients with limited respiratory function and the location of tumors render the above approaches of obtaining biopsy useless. These unfortunate problems can be resolved with the cooperation of tumor markers in multiple patients. Neuron-specific enolase (NSE) as a traditional serum biomarker for the diagnosis of SCLC, has been widely used in clinical practice, but its specificity is not always satisfactory due to many patients with NSCLC having raised NSE concentrations (9). Likewise, higher NSE serum levels are found in hemolytic specimens (9,10). Therefore, the discovery of more reliable diagnosis tumor markers to compensate for the deficiency of NSE is urgently needed. ProGRP is rarely expressed in normal human adult tissues, except in placental and embryonic tissues; it is more stable in the peripheral blood and not disturbed by hemolysis, except for renal dysfunction. Thus, many researches have suggested that ProGRP has a higher specificity. Still, no consensus has been reached concerning the discrepancy in the sensitivity to SCLC of NSE and ProGRP (11,12). A review of the literature indicates that ProGRP’s concentration is closely related to the cancer stage (13-15); however, there is no published research about whether the cut-off values of ProGRP change with different stages in the diagnosis of SCLC. Moreover, ProGRP’s diagnostic efficacy for LCNEC, typical carcinoid and atypical carcinoid (TC and AC), and type of pulmonary neuroendocrine tumor (PNET) as SCLC, still remain unclear. Previous research observed that high ProGRP level was also found in patients with LCNEC, TC and AC (13,16-18). Therefore, we retrospectively analyzed data to investigate the following: (I) the differences in the sensitivity and specificity of ProGRP versus NSE in the diagnosis of SCLC and other PNETs; and (II) the values of ProGRP and NSE in diagnosing SCLC in addition to other PNETs in lung cancer patients with different stages.
Methods
Study population
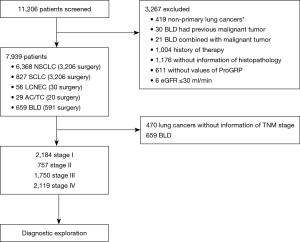
We retrospectively analyzed 11,206 patients under clinical suspicion of lung cancer from January 1, 2015 to May 31, 2018 in Tianjin Medical University Cancer Institute and Hospital. Patients included in this study had no previous malignant tumors and without history of antineoplastic therapy. Other inclusion and exclusion criteria are summarized in Figure 1. Ultimately, 659 patients were confirmed to be benign lung disease (BLD), including infectious diseases [340] and noninfectious diseases [319]. In accordance with the 2015 WHO classification (19), lung cancers were diagnosed by histopathology and were categorized as NSCLC [including: adenocarcinoma (4,492 cases), squamous cell carcinoma (1,461 cases), adenosquamous carcinoma (39 cases), large cell carcinoma (76 cases), sarcomatoid carcinoma (59 cases), salivary gland-type tumor (26 cases), NSCC NOS (215 cases)], SCLC (827 cases, including 70 cases combined small cell carcinoma), LCNEC (56 cases) and pulmonary carcinoid tumor (29 cases). Tumor staging was defined according to the recommendations of the “Eight Edition AJCC Cancer Staging Manual” [2017] and the Veterans Administration Lung Cancer Group A Staging System.
Data collection
The serum levels of tumor markers were analyzed prior to treatment while patients were at the fasting state. Blood samples of about 4 mL were obtained by venous puncture and centrifuged at 3,000 rpm. Assay for serum NSE and plasma ProGRP were measured by electrochemiluminescence immunoassay analyzer (Roche, Germany). The upper limit value (ULV) in the normal range of NSE and ProGRP was 15.2 µg/L and 63 ng/L respectively.
Statistical analysis
Statistical analyses were done with SPSS for Windows (version 22.0) and MedCalc (version 15.2.2). Data were expressed as median and interquartile range (IQR). The incidence of different pathological types was calculated as fractions in this study. Continuous variables were tested with the Kruskal-Wallis and Mann-Whitney U test. The Chi-squared and Fisher exact tests were used to compare categorical data, and confidence intervals of proportions were calculated with the Wald (asymptotic) method. Sensitivity, specificity, areas under the curves (AUCs) and optimal cutoff value were calculated by operating characteristic curve (ROC). Delong’s test was used to compare the difference of AUCs. Scatter plots were constructed to indicate the relationship between pathological types and the levels of tumor markers, with the use of GraphPad Prism 5 software. P<0.05 was considered statistically significant.
Results
The levels of tumor markers in different lung tumors
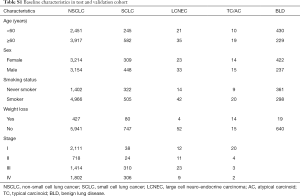
After excluding patients using the selection criteria (Figure 1), 7,939 cases were deemed eligible for this study. Clinicopathological characteristics of patients are summarized in Table S1.
Full table
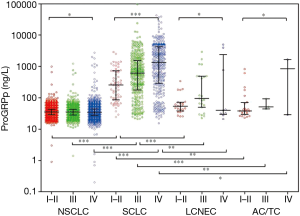
The distribution of ProGRP and NSE in patients is shown in Figures 2,S1; compared to the BLD, the ProGRP level was significantly higher in lung cancer patients (median 32.6 vs. 36.7 ng/L, P<0.001), while slightly elevated ProRGP levels were found in BLD compared to NSE (2.0% vs. 6.2%). In all patients with lung tumors, the highest level of ProGRP was in SCLC (median 32.6 ng/L), then LCNEC (median 32.6 ng/L), followed by NSCLC (median 32.6 ng/L) and TC/AC (median 32.6 ng/L) (P<0.001). It was interesting to note that ProGRP seem to be abnormally higher in poorly-differentiated neuroendocrine carcinoma (PD-NEC containing SCLC and LCNEC) than well-differentiated neuroendocrine tumor (WD-NET containing TC and AC) and NSCLC (Figure 2). Although the distribution trend of NSE was similar to ProGRP in four lung tumor cohorts, the false positive rate of NSE (27.3%) was significantly higher than that of ProGRP (4.3%) in NSCLC (Figures 2,S1).


Diagnosis value of tumor markers
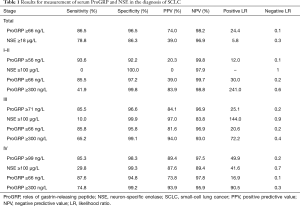
In this study, ROC showed that an optimum diagnostic cutoff for ProGRP and NSE was 66 ng/L and 18 µg/L respectively, for the diagnosis of SCLC. Furthermore, at this cutoff value, the sensitivity (86.5% vs. 78.8%) and specificity (96.5% vs. 86.3%) of ProGRP were higher than NSE for the diagnosis of SCLC. In addition, ProGRP had a greater AUC value than did NSE in patients with SCLC (0.943 vs. 0.894, 95% CI: 0.033–0.065, P<0.001). Furthermore, both the positive predictive value (PPV) and negative predictive value (NPV) of ProGRP were higher than NSE (Table 1). With the assessment of AUC, sensitivity, specificity, PPV, NPV and likelihood ratios, ProGRP had a greater differential diagnostic accuracy than NSE. These results were similar to the diagnostic efficacy of NEC, while ProGRP had a better diagnostic performance than NSE (Table S2).
Full table

Full table
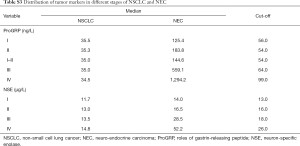
Diagnosis value of tumor markers in different stages
Figures 3,S2 shows the distribution of ProGRP and NSE in lung cancer patients, subdivided according to histology and staging. A positive correlation was found between ProGRP’s concentration and stage in SCLC patients (P<0.001) (Figure 3). We used this finding as a basis to explore whether the cut-off of ProGRP varies with different stages. Results obtained from the analysis of cut-off are presented in Table 2; it can be seen that the cutoff values for SCLC patients with stage I and II were similar, and the number of the two cohorts were both small. Therefore, all patients were divided into three groups of I–II, III and IV. ROC indicated that in stage I–II, III and IV, the optimum diagnostic cutoffs for ProGRP to SCLC were 56.0 ng/L (AUC 0.955), 71.0 ng/L (AUC 0.933), and 99.0 ng/L (AUC 0.951) respectively. ProGRP had greater AUC (I–II: 95% CI: 0.133–0.285, P<0.001; III: 95% CI: 0.036–0.078 P<0.001; IV: 95% CI: 0.029–0.080, P<0.001), sensitivity, specificity, PPV and NPV values than did NSE in SCLC patients with stage I–II <0.001), Table 1). The greater diagnostic performance of ProGRP to NSE was similar to that showed in NEC (Tables S2,S3; Figure S3).


Full table
Full table

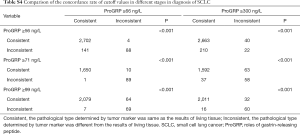
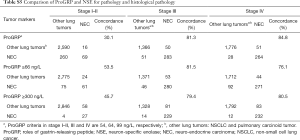
It can be seen that diagnostic efficacy of ProGRP is superior to NSE in the diagnosis of SCLC in different stages. However, it is still unknown whether the diagnostic performances of using different cutoff values were superior to using ProGRP ≥66 ng/L in different stages. Moreover, the criteria of ProGRP ≥300 ng/L for SCLC, without interference of impaired renal function, was recommended by the European Federation of Clinical Chemistry and Laboratory Medicine (CCLM). Further statistical analysis was performed to discuss the diagnostic discrepancy among them. As can be seen from Table 1, the higher value of ProGRP, the higher the specificity, PPV, and LR (positive), while the lower the sensitivity, NPV, and LR (negative) in the diagnosis of SCLC. Besides, Tables 3,S4 present that in lung tumors patients with stage III and IV, the combined positive and negative concordance rates of ProGRP ≥71 ng/L, and ProGRP ≥99 ng/L were better than ProGRP ≥66 ng/L (χ2 =1,526.9 and 988.7, both P<0.001) and ProGRP ≥300 ng/L (χ2 =448.6 and 137.9, both P<0.001). Meanwhile, in stage I–IIile, in stage I both an PrProGRP ≥56 ng/L was inferior to the other criteria [ProGRP ≥66 ng/L (χ2 =1,006.3, P<0.001) and ProGRP ≥300 ng/L (χ2 =62.4, P<0.001)]. In addition, the concordance rate of ProGRP ≥300 ng/L was higher than ProGRP ≥66 ng/L (χ2 =121.5, P<0.001). Finally, there was no evidence that the concordances of different cutoff values in the diagnosis of NEC with different stages were superior to ProGRP ≥66 ng/L and ProGRP ≥300 ng/L (Tables S5,S6).

Full table
Full table
Full table

Full table
Discussion
In this study, ProGRP was observed to be superior to NSE in the diagnosis of SCLC patients with all stages. While the level of ProGRP did progress with the stage, there was no significant difference in the different stages of NSCLC. With respect to improving the accuracy of the diagnosis of SCLC, we further explored the diagnostic efficacy of the different thresholds at different stages. Finally, the consistency of diagnosis was found to be improved by using different cutoff values in different stages, especially in stage III and IV. In regards to the criteria of ProGRP ≥56 ng/L not having a higher concordance rate than other criteria in stage I–II, it may be that the proportion of SCLC (2.1%, 62/2,938) with stage I–II, was far less than that with stage III (17.7%, 310/1,750) and IV (14.4%, 306/2,119). There are two possible explanations for this result: the first is that the number of SCLC patients with early stage was more than those with late stage (20,21), and the second is that ProGRP in patients with extensive disease were significantly higher than those with limited disease (14,22). Although 7.3% (207/2,826) of NSCLC patients were erroneously diagnosed as SCLC, the sensitivity (93.6%) of diagnosis to SCLC was further improved to detect more SCLC with early stage. Furthermore, from the analysis of data in this study, ProGRP’s diagnosis efficacy was also superior to NSE in different stages.
In the current work, PNET patients shared elevated ProGRP levels, and the most novel finding was that high levels of ProGRP appear to be associated with PD-NEC, whereas WD-NET patients had much lower ProGRP levels. However, previous research had only realized that PNET patients share elevated ProGRP levels (17,18,23,24), while the difference among them was not further clarified, along with the diagnosis value of ProGRP for PNET is also undetermined. The purpose of the present study was to determine the efficacy of ProGRP for PNET. As for the awareness of ProGRP, it is limited to its precursor molecule, GRP, which has been identified in the neuroendocrine cells of the lungs of fetuses (25-27). However, the potential mechanism remains unclear. There are studies that have indicated that the inhibition of ProGRP depresses NE cell proliferation and promotes apoptosis (25). It can be seen that ProGRP appears to be involved in the rapid cell growth of PD-NEC (28-33), which may seem to cause differences in the expression of ProGRP in different PNETs. However, further studies are required to detect the mechanisms of ProGRP differential expression in PNET from the perspective of genes and proteins in order for them to lead to a potential treatment for SCLC. According to the distributions of ProGRP in four lung tumor cohorts, the data was analyzed to explore ProGRP’s efficacy in the diagnosis of NEC. The cutoff value was the same as it was for SCLC and also shared preferred sensitivity and specificity (Table S2), and different cutoff values are not recommended for diagnosis in different stages.
In conclusion, ProGRP has a higher sensitivity and specificity compared to NSE in the diagnosis of SCLC and NEC. A limitation of this study is that our data were collected and reviewed retrospectively and prospective studies are thus warranted to examine the efficacy of ProGRP in different stages. However, we believe that the implementation of different ProGRP cutoff values in the diagnosis of SCLC with different stages can further improve the diagnostic accuracy. As a specific tumor marker associated with SCLC, ProGRP deserves more research attention in order explore its value in the diagnosis and prognosis prediction of SCLC and prepare it for widespread use in clinical practice.
Acknowledgements
Funding: This work was supported by National Key Technology R&D Program (2015BALL12B12).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Meuwissen R, Linn SC, Linnoila RI, et al. Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell 2003;4:181-9. [Crossref] [PubMed]
- Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer 2015;121:664-72. [Crossref] [PubMed]
- Iyoda A, Hiroshima K, Moriya Y, et al. Postoperative recurrence and the role of adjuvant chemotherapy in patients with pulmonary large-cell neuroendocrine carcinoma. J Thorac Cardiovasc Surg 2009;138:446-53. [Crossref] [PubMed]
- Besse B, Adjei A, Baas P, et al. 2nd ESMO Consensus Conference on Lung Cancer: non-small-cell lung cancer first-line/second and further lines of treatment in advanced disease. Ann Oncol 2014;25:1475-84. [Crossref] [PubMed]
- Manzo A, Montanino A, Carillio G, et al. Angiogenesis Inhibitors in NSCLC. Int J Mol Sci 2017;18. [Crossref] [PubMed]
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Inoue A, Kobayashi K, Usui K, et al. First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol 2009;27:1394-400. [Crossref] [PubMed]
- Liu SV, Camidge DR, Gettinger SN, et al. Long-term survival follow-up of atezolizumab in combination with platinum-based doublet chemotherapy in patients with advanced non-small-cell lung cancer. Eur J Cancer 2018;101:114-22. [Crossref] [PubMed]
- Dong Y, Zheng X, Yang Z, et al. Serum carcinoembryonic antigen, neuron-specific enolase as biomarkers for diagnosis of non-small cell lung cancer. J Cancer Res Ther 2016;12:34-6. [Crossref] [PubMed]
- Berger R, Richichi R. Derivation and validation of an equation for adjustment of neuron-specific enolase concentrations in hemolyzed serum. Pediatr Crit Care Med 2009;10:260-3. [Crossref] [PubMed]
- Stieber P, Dienemann H, Schalhorn A, et al. Pro-gastrin-releasing peptide (ProGRP)--a useful marker in small cell lung carcinomas. Anticancer Res 1999;19:2673-8. [PubMed]
- Miyake Y, Kodama T, Yamaguchi K. Pro-gastrin-releasing peptide(31-98) is a specific tumor marker in patients with small cell lung carcinoma. Cancer Res 1994;54:2136-40. [PubMed]
- Wojcik E, Kulpa JK. Pro-gastrin-releasing peptide (ProGRP) as a biomarker in small-cell lung cancer diagnosis, monitoring and evaluation of treatment response. Lung Cancer (Auckl) 2017;8:231-40. [Crossref] [PubMed]
- Kim HR, Oh IJ, Shin MG, et al. Plasma proGRP concentration is sensitive and specific for discriminating small cell lung cancer from nonmalignant conditions or non-small cell lung cancer. J Korean Med Sci 2011;26:625-30. [Crossref] [PubMed]
- Oh HJ, Park HY, Kim KH, et al. Progastrin-releasing peptide as a diagnostic and therapeutic biomarker of small cell lung cancer. J Thorac Dis 2016;8:2530-7. [Crossref] [PubMed]
- Korse CM, Holdenrieder S, Zhi XY, et al. Multicenter evaluation of a new progastrin-releasing peptide (ProGRP) immunoassay across Europe and China. Clin Chim Acta 2015;438:388-95. [Crossref] [PubMed]
- Taira N, Kawabata T, Ichi T, et al. Utility of the serum ProGRP level for follow-up of pulmonary carcinoid tumors. Am J Case Rep 2014;15:337-9. [Crossref] [PubMed]
- Korse CM, Taal BG, Bonfrer JM, et al. An elevated progastrin-releasing peptide level in patients with well-differentiated neuroendocrine tumours indicates a primary tumour in the lung and predicts a shorter survival. Ann Oncol 2011;22:2625-30. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Imai H, Mori K, Watase N, et al. Clinical Significance of the Relationship between Progression-Free Survival or Postprogression Survival and Overall Survival in Patients with Extensive Disease-Small-Cell Lung Cancer Treated with Carboplatin plus Etoposide. Can Respir J 2016;2016:5405810. [Crossref] [PubMed]
- Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539-44. [Crossref] [PubMed]
- Peng Y, Wang Y, Li J, et al. Utility of NSE, ProGRP and LDH in Diagnosis and Treatment in Patients with Small Cell Lung Cancer. Zhongguo Fei Ai Za Zhi 2016;19:590-4. [PubMed]
- Molina R, Auge JM, Bosch X, et al. Usefulness of serum tumor markers, including progastrin-releasing peptide, in patients with lung cancer:correlation with histology. Tumour biology 2009;30:121-9. [Crossref] [PubMed]
- Kudo K, Ohyanagi F, Horiike A, et al. Clinicopathological findings of non-small-cell lung cancer with high serum progastrin-releasing peptide concentrations. Lung Cancer 2011;74:401-404. [Crossref] [PubMed]
- Dumesny C, Patel O, Lachal S, et al. Synthesis, expression and biological activity of the prohormone for gastrin releasing peptide (ProGRP). Endocrinology 2006;147:502-9. [Crossref] [PubMed]
- Yamaguchi K. Production and Molecular Size Heterogeneity of Immunoreactive Gastrinreleasing Peptide in Fetal and Adult Lungs and Primary Lung Tumors. Cancer Res 1983;43:3932-9. [PubMed]
- Ischia J, Patel O, Shulkes A, et al. Gastrin- Gastrin Lungs and peptide:Different forms, different functions. Biofactors 2009;35:69-75. [Crossref] [PubMed]
- Klimstra DS. Pathologic Classification of Neuroendocrine Neoplasms. Hematol Oncol Clin North Am 2016;30:1-19. [Crossref] [PubMed]
- Fernandez-Cuesta L, McKay JD. Genomic architecture of lung cancers. Curr Opin Oncol 2016;28:52-7. [Crossref] [PubMed]
- George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015;524:47-53. [Crossref] [PubMed]
- Derks JL, Hendriks LE, Buikhuisen WA, et al. Clinical features of large cell neuroendocrine carcinoma: a population-based overview. Eur Respir J 2016;47:615-24. [Crossref] [PubMed]
- Miyoshi T, Umemura S, Matsumura Y, et al. Genomic Profiling of Large-Cell Neuroendocrine Carcinoma of the Lung. Clin Cancer Res 2017;23:757-65. [Crossref] [PubMed]
- Rekhtman N, Pietanza MC, Hellmann MD, et al. Next-Generation Sequencing of Pulmonary Large Cell Neuroendocrine Carcinoma Reveals Small Cell Carcinoma-like and Non-Small Cell Carcinoma-like Subsets. Clin Cancer Res 2016;22:3618-29. [Crossref] [PubMed]


