Novel risk model for predicting acute adverse drug reactions following cardiac catheterization from TRUST study (The Safety and toleRability of UltraviSt in Patients Undergoing Cardiac CaTheterization)
Introduction
Acute adverse drug reactions (ADRs) of contrast media are defined as abnormal symptoms occurring within 1 hour following the administration of contrast media during cardiac catheterization. All of these symptoms vary from mild reactions, such as nausea, vomiting, and headache, to severe reactions such as laryngeal edema, cardiac dysrhythmias, pulmonary collapse, and others that could be life threatening (1,2). Our previous work demonstrated that the incidence of ADRs related to iopromide use was quite low, with only 58 (0.38%) of 17,513 patients observed with mild ADRs, while merely 2 patients had severe reactions (3). The same was found with other contrast media, such as iobitridol and iodixanol (4,5). However, uncommon as they were, based on the number of 75 million patients who undergo percutaneous coronary intervention (PCI) or coronary angiography every year, quite a lot of patients would suffer from undesirable ADRs, some of which are even fatal. Therefore, it is still necessary to reduce the rate of ADRs to as low as possible. Premedication with corticosteroids and antihistamines is efficient in preventing ADRs (6,7). Although emergent treatments for the ADRs usually work, some severe and fatal reactions, most of which occur within 20 min of the contrast medium injection, are too sudden and changeable to deal with (8). Early recognition of patients with a high risk of ADRs and premedication would be better to avoid the adverse reactions. History of previous ADRs, contrast media type, and age were all reported to be risk factors of ADRs (9-11); however, a comprehensive tool that includes all risk factors to stratify the risk level of ADRs does not exist. Hence, on the base of TRUST trial(The Safety and toleRability of UltraviSt in Patients Undergoing Cardiac CaTheterization, ClinicalTrials.gov identifier: NCT01206257), we aim to develop a simple risk score model to be applied by clinicians at bedside to evaluate the risk of developing ADRs, so that action can be taken before unexpected reactions occur.
Methods
We enrolled a cluster of consecutive patients between August 2010 and September 2011 in the TRUST study (The Safety and toleRability of UltraviSt in Patients Undergoing Cardiac CaTheterization). All patients who underwent coronary angiography and/or PCI according to the PCI guidelines (12) were eligible. We excluded pregnant and lactating women and patients with contraindications to iopromide or cardiac catheterization. As for the unified setting, patients accepted iopromide 300 or 370 mg/mL (Ultravist; Bayer Healthcare, Berlin, Germany) during the procedures without exception.
All data on adverse events (AEs) were recorded on the case report form by the investigator, including the incidence, seriousness, duration, action taken, and outcome. The final judgment of which AEs should be defined as ADRs was done by either the investigators or the study sponsor, Bayer HealthCare Company Ltd.
According to the American College of Radiology criteria (13), ADRs were defined as adverse reactions occurring within 1 hour after the injection of contrast media. The severity of the ADR was classified as mild, moderate, or severe. A mild ADR was defined as self-limited adverse reactions without evidence of progression and usually requiring no treatment. Moderate ADRs were not immediately life threatening (although they might progress to be so) but often required treatment. A severe ADR was potentially or immediately life threatening and prompt recognition and treatment were required. In addition, an ADR that resulted in death, threatened life, required inpatient hospitalization or prolongation of existing hospitalization, or led to any other events that do not fit the other outcomes but that jeopardized the patient and might require medical or surgical intervention (treatment) to prevent one of the other (serious) outcomes was defined as a serious adverse reaction (SAE). SAEs were all reported to the local drug safety manager within 24 hours, and the outcomes of all SAEs were followed up as well as documented. All ADRs were coded according to the Medical Dictionary for Regulatory Activities (14) and recorded on the case report form. All data were collected by trained personnel prospectively, and the occurrence of acute ADRs was centrally reviewed and categorized by the coordinating project management team (H&J CRO International, Inc.). Moreover, the primary committee performed the final check on the database to ensure quality. The methods of data extraction and management have been described in more detail previously (3).
Risk model development
We randomly divided the 17,139 patients into development and validation groups in a 2:1 manner, respectively. The data set of the development group was used to identify the univariate associations between baseline and key procedural characteristics and ADRs by Student t-test, chi-square test, or Fisher’s exact test. Next, multivariate logistic regression analysis was performed to identify independent predictors of ADRs and to estimate odds ratios. The significant risk factors identified in the univariate analysis were selected for the final model. We set the predictive score of each risk factor based on the β regression coefficient values accordingly. To provide the facilitated bedside assessment of ARD risk, we stratified the risk level as low, moderate, and high according to the risk score calculated for each individual. Then, discrimination and calibration of this risk model were assessed to evaluate the predictive performance. The receiver-operating characteristic curve was drawn to obtain the concordance index (c-index), which indicated the discrimination. The calibration of the model was examined by Hosmer-Lemeshow goodness-of-fit test. Finally, both data sets of the development group and validation group were used to calculate the incidence of ADRs according to each risk score and risk level, respectively, for the purpose of examining the efficiency and conformance of the risk scoring model both groups’ data sets.
Results
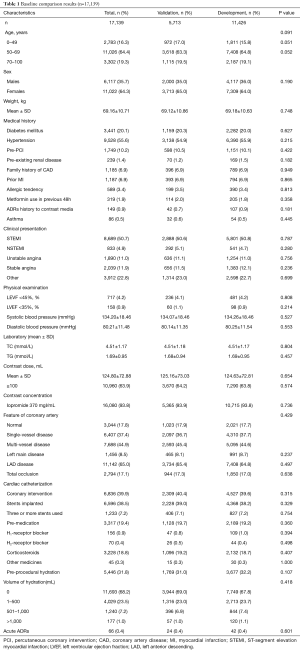
A total of 17,139 patients were included in the risk model–developing study, and patients were randomly assigned into the development group (n=11,426) and validation group (n=5,713) in 2:1 manner. Baseline characteristic were shown in Table 1. All the participants were Chinese without foreigner. The baseline demographic and clinical characteristics are listed in Table 1. Briefly, patients aged 50 to 69 years accounted for the majority at about 64.3%; 35.7% of the gross population were male patients. All patients used iopromide as contrast media; 93.8% used a concentration of 370 mgI/mL. Forty-two patients experienced ADRs in the development group, while 24 patients experienced ADRs in the validation group. The incidence (0.4%) of ADRs was approximately even in both groups. Generally speaking, there were no statistically significant differences between the development and validation groups. All kind of ADRs were listed in Table 2.
Full table
Full table
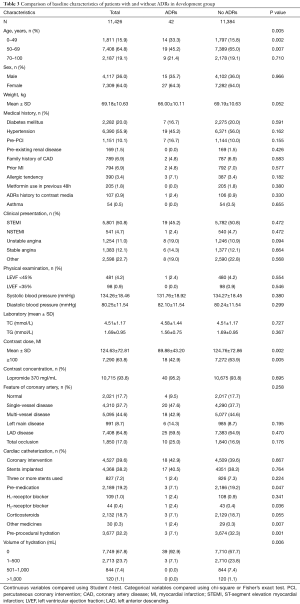
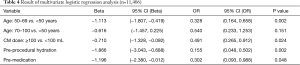
Baseline characteristic comparisons were listed out between patients with and without ADRs. As shown in Table 3, age, contrast media dose (≥100 mL), premedication, and preprocedural hydration were significant variables correlated with ADRs. The estimated odds ratios and confidence intervals of the predictive factors are shown in Table 4. As can be seen, the odds ratios of these factors were less than 1, which indicated that age (50–69 years), contrast media dose ≥100 mL, preprocedural hydration, and premedication were protective factors for ADRs. Hence, it is reasonable for us to consider that age (except for 50–69 years), contrast media dose <100 mL, preprocedural hydration absence, and premedication absence are factors correlated with a higher risk of ADRs. Based on the β regression coefficient values, we set the risk scores according to the corresponding variables on the weight as follows:
Full table
Full table
- Age: if not 50–69 years, score =1;
- Contrast media dose <100, score =1;
- Preprocedural hydration: if not, score =2;
- Premedication: if not, score = 1.
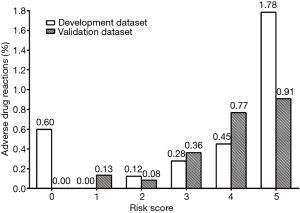
The risk score formula was RS (risk score) = 1 (age not 50–69) + 1 (CM dose <100) + 2 (preprocedural hydration: not) + 1 (premedication: not). Furthermore, we categorized the patients with different risk scores into graduated risk levels according to the predicted probability of ADRs: low risk, score 0–2 (predicted probability: 0.09%); moderate risk, score 3–4 (predicted probability: 0.36%); high risk, score ≥5 (predicted probability: 1.78%).
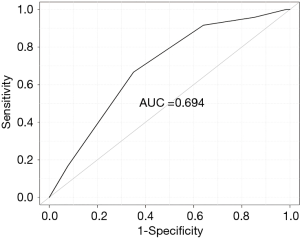
The receiver-operating characteristic curve is shown in Figure 1, which indicates the risk model is moderately discriminatory with a concordance index of 0.694. The chi-square value of the Hosmer-Lemeshow goodness-of-fit test was 9.461 (P=0.305), which shows adapted calibration of this predictive model.
Finally, on the basis of this risk-scoring model, the incidences of ADRs of corresponding different risk scores and risk levels are shown in Figures 2 and 3, including both development group and validation group data sets. Basically, the risk of ADR occurrence progresses as the risk score increases from 1 to 5 and as the risk level elevates. The rate of ADRs was similar between the development group and validation group. Generally, the risk-scoring model derived from the development data set predicts the same tendency in the validation data set.

Discussion
Here we study the incidence of ADRs among 17,139 patients who underwent PCI. Moreover, we developed a simplified algorithm to predict the risk probability of AE occurrence. As shown above, age, dose of contrast media, preprocedural hydration, and premedication are key factors that account for the prediction of ADRs.
Age has been widely discussed for its importance to ADR prediction. Kopp et al. and Vogl et al. (4,10) found that age 18–30 years was associated with a higher incidence of ADRs. Another study conducted by Lasser et al. (15) considered that patients aged between 20 and 50 years had a higher probability of ADR occurrence, while patients either younger than 20 years or older than 50 years had a reduced probability of ADR occurrence. The postmarketing surveillance study (5) with iodixanol reported that patients younger than 65 years had an increased risk of ADRs. Nevertheless, according to our study, we found that patients between the ages of 50 and 69 have a reduced risk of ADRs; patients aged younger than 50 or older than 65 are assigned a score of 1 in the algorithm, which corresponds to an increased risk of ADR occurrence. The variable conclusions about the risked age segment may be due to the different populations and contrast media type. Most of the patients in this study used iopromide as the contrast media during the PCI. In addition, the contrast media were injected directly into the coronary artery rather than through a vein or peripheral artery. Therefore, with intracoronary artery use of iopromide, patients who are younger than 50 years or older than 65 years should pay more attention to the possibility of ADRs.
Contrast media dose is a common risk factor related to contrast-induced nephropathy (CIN), and the probability of CIN increases proportionally with the increase in volume of contrast media (16). However, it is not clear how contrast media volume influences ADRs. In this study, we found that a contrast media dose less than 100 mL was associated with a higher possibility of ADRs, which differs from the accepted logic of how contrast media affects CIN. As reported (9), ADRs were more or less associated with anaphylaxis. Therefore, the probable explanation is that more contrast media contact increases the tolerance of the body to contrast media, which could cause less anaphylaxis and fewer ADRs as a result.
Prehydration and premedication were the other two risk factors in this model. Especially important was prehydration, which accounts for a score of 2 if absent. It has been well documented that hydration minimizes, or decreases, the incidence of ADRs induced by CM. Though unclear, it seems plausible that adequate hydration may counteract some of the putative hemodynamic effects leading to contrast-induced adverse effects and CIN, a common risk factor associated with lack of pre-hydration (17,18). On the other hand, pre-hydration mediated increase in total body fluid volume, thereby may reduce the concentration of CM and thus prevent ADRs. This explains the finding in current model wherein absence of pre-procedural hydration increases the risk of moderate to severe ADRs.
Corticosteroids and H1/H2-receptor blocker were the common treatment for patients with adverse drug reactions. For patients who were evaluated to be high risk of ADRs, prophylactic premedication prior to administration of CM is proposed to be most effective in reducing the occurrence of mild or moderate ADRs. Absence of pre-medication as seen in the present study would therefore increase the risk of ADR.
Moreover, some other studies have considered the history of ADRs as a significant risk factor of the next ADR occurrence (19,20). However, we found it statistically insignificant enough to include history of ADRs as a predictive factor of the algorithm. Insufficient case reports of patients may be one of the underlying reasons, and more detailed and comprehensive studies are needed to further improve the accuracy and efficacy of the predictive model.
Study limitation
Reports of mild ADR are mostly based on patients’ subjective opinions, which may result in subjective bias during the analysis. On the other hand, only 2 severe ADRs and 3 serious ADRs were reported. Therefore, the prediction of severe or serious ADRs may lack appropriate efficiency. More observations of ADRs are needed for development of a better predicted algorithm.
Conclusions
We have developed a predictive model of ADRs following PCI and established an algorithm to estimate the probability of ADR occurrence. Age, contrast media dose, prehydration, and premedication are the basic factors of the model, as indicated by proper efficacy and accuracy.
Acknowledgements
Funding: The TRUST study was funded by Bayer Pharma AG, Germany and Science and Technology Planning Project of Guangdong Province (2014B070706010), National Science Foundation for Young Scientist of China (grant No. 81500520), The Progress in Science and Technology Project of Guangdong Province (grant No. 2015A030302037), Guangdong Provincial Medical Research Fund Project (GSIC20140526), and Guangdong Provincial People’s Hospital Clinical Transformation Research Project (2015zh01).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Guangdong General Hospital Ethics Committee and written informed consent was obtained from all patients.
References
- Thomsen HS, Morcos SK. Management of acute adverse reactions to contrast media. Eur Radiol 2004;14:476-81. [Crossref] [PubMed]
- Beckett KR, Moriarity AK, Langer JM. Safe Use of Contrast Media: What the Radiologist Needs to Know. Radiographics 2015;35:1738-50. [Crossref] [PubMed]
- Chen JY, Liu Y, Zhou YL, et al. Safety and tolerability of iopromide in patients undergoing cardiac catheterization: real-world multicenter experience with 17,513 patients from the TRUST trial. Int J Cardiovasc Imaging 2015;31:1281-91. [Crossref] [PubMed]
- Vogl TJ, Honold E, Wolf M, et al. Safety of iobitridol in the general population and at-risk patients. Eur Radiol 2006;16:1288-97. [Crossref] [PubMed]
- Zhang BC, Hou L, Lv B, et al. Post-marketing surveillance study with iodixanol in 20 185 Chinese patients from routine clinical practices. Br J Radiol 2014;87:20130325. [Crossref] [PubMed]
- Morcos SK. Review article: Acute serious and fatal reactions to contrast media: our current understanding. Br J Radiol 2005;78:686-93. [Crossref] [PubMed]
- Radhakrishnan S, Manoharan S, Fleet M. Repeat survey of current practice regarding corticosteroid prophylaxis for patients at increased risk of adverse reaction to intravascular contrast agents. Clin Radiol 2005;60:58-63; discussion 56-7. [Crossref] [PubMed]
- Shehadi WH. Death following intravascular administration of contrast media. Acta Radiol Diagn (Stockh) 1985;26:457-61. [Crossref] [PubMed]
- Palkowitsch P, Lengsfeld P, Stauch K, et al. Safety and diagnostic image quality of iopromide: results of a large non-interventional observational study of European and Asian patients (IMAGE). Acta Radiol 2012;53:179-86. [Crossref] [PubMed]
- Kopp AF, Mortele KJ, Cho YD, et al. Prevalence of acute reactions to iopromide: postmarketing surveillance study of 74,717 patients. Acta Radiol 2008;49:902-11. [Crossref] [PubMed]
- Sutton AG, Finn P, Campbell PG, et al. Early and late reactions following the use of iopamidol 340, iomeprol 350 and iodixanol 320 in cardiac catheterization. J Invasive Cardiol 2003;15:133-8. [PubMed]
- Patel MR, Dehmer GJ, Hirshfeld JW, et al. ACCF/SCAI/STS/AATS/AHA /ASNC 2009 Appropriateness Criteria for Coronary Revascularization: a report by the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology Endorsed by the American Society of Echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 2009;53:530-53. [Crossref] [PubMed]
- ACR Committee on Drugs and Constrast Media (2010) ACR manual on contrast media version 7. Available online: http://www.acr.org/
- Medical Dictionary for Regulatory Activities (MedDRA) Version 13.0. March 2010. Available online: http://www.meddra.org
- Lasser EC, Berry CC, Mishkin MM, et al. Pretreatment with corticosteroids to prevent adverse reactions to nonionic contrast media. AJR Am J Roentgenol 1994;162:523-6. [Crossref] [PubMed]
- Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol 2004;44:1393-9. [PubMed]
- Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis 2002;39:930-6. [Crossref] [PubMed]
- Fähling M, Seeliger E, Patzak A, et al. Understanding and preventing contrast-induced acute kidney injury. Nat Rev Nephrol 2017;13:169-80. [Crossref] [PubMed]
- Morcos SK, Thomsen HS, Webb JA. Prevention of generalized reactions to contrast media: a consensus report and guidelines. Eur Radiol 2001;11:1720-8. [Crossref] [PubMed]
- Park HJ, Park JW, Yang MS, et al. Re-exposure to low osmolar iodinated contrast media in patients with prior moderate-to-severe hypersensitivity reactions: A multicentre retrospective cohort study. Eur Radiol 2017;27:2886-93. [Crossref] [PubMed]