
Pleurectomy/decortication and hyperthermic intrathoracic chemoperfusion using cisplatin and doxorubicin for malignant pleural mesothelioma
Introduction
Malignant pleural mesothelioma (MPM) is a rare and highly aggressive malignancy of the pleural cavity, mainly associated with exposure to asbestos. The widespread use of asbestos in past decades together with the long latency of MPM are responsible for the still increasing incidence of MPM (1), affecting 7–40 people per million inhabitants depending on the geographic region (2). Symptom-based findings present at a late stage of the disease. In the absence of effective screening methods, the detection of MPM at early stages is rare. Extensive local tumor burden infiltrating into the chest wall or the mediastinum is often present at diagnosis of MPM (3). Despite current advances in chemotherapy for mesothelioma treatment, median survival for unresectable disease ranges between 4 and 13 months (3,4). Hence, multimodal treatment regimens will be important to increase survival for these patients.
Because of late occurring symptoms and diagnosis at an advanced stage, only few patients are eligible for a multimodal treatment approach (3,4). Moreover, due to the laminar tumor growth within the entire pleura, surgery alone is not able to achieve microscopic complete (R0) resection. Therefore, combined treatment modalities have been established in many centres during the last years to achieve a better local tumor control with increasing overall survival (2,5). For example, localized MPM in patients with a good performance status has been treated within a multimodal concept including neoadjuvant chemotherapy, extrapleural pneumonectomy (EPP) and adjuvant radiotherapy to increase life expectancy (6,7).
MPM is mainly diagnosed in older patients with reduced physical condition and increased comorbidities. Since only few patients were eligible for EPP, potential surgical treatment concepts for localized MPM have been evaluated during the last years with focus on pleurectomy/decortication (5-9). In order to destroy remaining tumor cells, hyperthermic intrathoracic chemoperfusion of the thoracic cavity (HITHOC) after pleurectomy and decortication (P/D) leads to increasing overall survival rates with low postoperative morbidity and mortality (6,9). Consequently, P/D is the treatment of choice for localized disease (5-9).
In the present study, we retrolectively evaluated our experience with P/D followed by HITHOC for localized mesothelioma.
Methods
Patients
From 11/2009 until 11/2013, 71 patients with MPM underwent P/D and HITHOC at a single center, the Lung Clinic Gauting. We retrospectively analyzed the patient data from medical charts and acquired the follow-up information from medical charts and by telephone. The study was approved by the Ethics Committee of the Ludwig-Maximilians-University of Munich (No. 711-16).
Preoperative assessment
Histopathologic confirmation and histological subtyping of MPM was performed by multiple pleural biopsies via single port thoracoscopy. The existing macroscopic tumor mass was estimated during this operation. Preoperative investigations were conducted according to the guidelines of the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS) for the management of MPM (10). Exclusion of distant metastases was performed using positron emission tomography (PET), while infiltration of important local structures like chest wall and mediastinum was analyzed by magnetic resonance imaging. All patients underwent functional respiratory and cardiac testing using ergospirometry, lung scintigraphy, and, depending on the findings, echocardiography. In conjunction with these tests, predicted postoperative function parameters [forced expiratory volume in one second (FEV1), forced vital capacity (FVC), DLCO and cardio-respiratory reserve (VO2max) were calculated. Based on the recommendations for pneumonectomy in lung cancer surgery, patients were excluded from surgery when FEV1 was <70% or VO2max was below 15 mL/min/kg. Due to possible side effects of chemotherapeutic drugs, patients having a left ventricular ejection fraction <45% were also disqualified from surgery. Mediastinoscopy or endobronchial ultrasound with transbronchial needle aspiration was only performed to further evaluate PET-positive lymph nodes of the N2 and N3 region (11,12). Each patient was discussed in our interdisciplinary tumor board to create an individual and multimodal treatment concept.
Surgical technique
Surgery was performed by three different, experienced thoracic surgeons. After anterolateral thoracotomy, P/D of the parietal and visceral pleura with respect to the fissures was performed. To achieve macroscopic complete resection with tumor burden below 1 cm3, extended P/D with resection of the diaphragm and/or pericardium was performed according to the definition of the International Mesothelioma Interest Group if necessary and possible (13). Otherwise, patients were classified as having macroscopic incomplete resection. Finally, we aimed at achieving an adequate expansion of the lung to avoid the high postoperative morbidity associated with persistent air leakage. Therefore, P/D of the visceral pleural was additionally focussed on the interlobar fissures and large air leakages were closed by sutures.
HITHOC
Five chest tubes were placed in the thoracic cavity before the anterolateral thoracotomy was closed. Hyperthermic perfusion was accomplished at 42 °C using RanD Performer HT (RanD S.r.l.; Medolla, Italy), a specialized device for hyperthermic intraperitoneal chemoperfusion and HITHOC. Intrathoracic temperature was reached after a median of 12.4 minutes (range, 8.3–19.6 minutes). Cisplatin (200 mg) and doxorubicin (100 mg) were added into the 5,000 mL 0.9% saline solution and HITHOC was performed for 90 minutes at 42 °C. As the amount of fluid present in the thoracic cavity varied from patient to patient, we decided to keep the concentration of both cytotoxic agents in the perfusate solution stable. For this reason, fixed concentrations per liter perfusion fluid have been calculated. Three probes controlled the temperature close to oesophagus, heart and pleural apex continuously.
Postoperative morbidity and follow-up
To prevent peri- and postoperative, and especially chemotherapy related complications, patients were monitored on our intensive care unit after surgery. Heart rate, blood pressure, arterial blood gas analysis and laboratory parameters for kidney, liver, electrolytes, heart, infection and blood count were collected according to a defined standard. For nephroprotection, mean arterial pressure was maintained above 60 mmHg. Volume infusions and diuretic therapy were controlled to achieve a diuresis of more than 50 mL per hour. Postoperative morbidity and mortality were analyzed retrospectively. Complications were classified according to the Clavien-Dindo classification for surgical complications (14). Furthermore, patient age, gender, histologic tumor type, extent of resection, neoadjuvant treatment, adjuvant therapy and overall survival were evaluated. Computed tomography and chest X-ray were performed alternately every three months for follow-up. No patient underwent additive chemotherapy in the early postoperative time course. Census of follow-up for long term overall and disease-free survival was set in 03/2018.
Statistics
Results are given as mean ± standard deviation (SD) and median (interquartile range, IQR), as indicated. Survival is given as median (interquartile range, IQR) and was examined by Kaplan-Meier analyses and log rank tests for comparisons. To analyze the variability of a parameter in the same individual the Wilcoxon signed rank test was used. P values <0.05 were considered to be statistically significant. Analyses were performed using Graph Pad Prism v 5.0 (GraphPad Software; San Diego, USA). SigmaPlot 13 (Systat Software Inc; Erkrath, Germany) was used for multivariate cox regression analysis.
Results
Patients
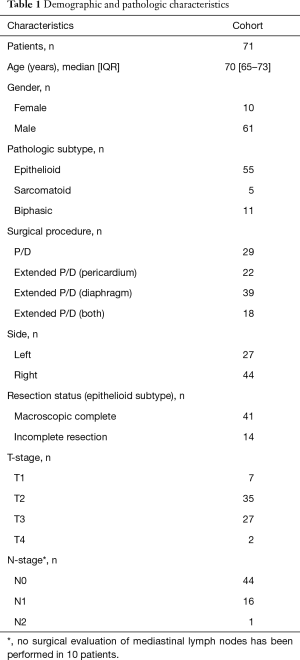
Seventy-one patients underwent P/D followed by HITHOC due to localized MPM and were analyzed retrospectively. There were 61 male and ten female patients with a median age of 70 years (range, 65–73 years). Forty-four patients had MPM on the right side and 27 patients on the left. Patient characteristics are summarized in Table 1. No patient presented cardiovascular disease, hepatic or renal function alterations before the operation. Video-assisted thoracoscopy with pleural biopsies was performed on all patients for histological confirmation of MPM. Talc pleurodesis was performed in 64 patients which was dependent on the individual hospital standard for thoracoscopy. Staging was carried out as described above to exclude contralateral or distant metastases. Eight patients had been previously treated with combination chemotherapy (cisplatin plus navelbine or cisplatin plus pemetrexed), including four patients with large tumor masses in the diaphragmatic region and four patients presenting at our hospital after chemotherapy for second opinion. Two patients showed tumor regression, six had stable disease.
Full table
Procedure
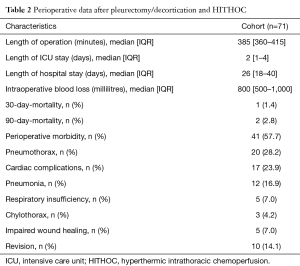
Median operating time was 385 minutes (range, 360–415 minutes), including 90 minutes of HITHOC at 42 °C using cisplatin and doxorubicin, as described above. During perfusion, four patients reacted with acute hypotension which could be handled by slowing down the perfusion rate and one patient showed a period of atrial fibrillation. Another patient suffered from short transient asystolia during the removal of the perfusion solution from the thoracic cavity which could be terminated by flow reduction. Postoperative stay in the intensive care unit was 2 days (range, 1–4 days) and overall hospital stay was 26 days (range, 18–40 days) (Table 2). Median intraoperative blood loss was 800 mL (range, 500–1,000 mL) and transfusion of 2 (range, 0–2) erythrocyte concentrates (300 mL each) was necessary. Perioperative morbidity was 57.7% with 41 patients suffering from 63 different complications detailed in Table 2.
Full table
Minor complications were present in 31 patients (43.7%). Twenty patients (28.2%) suffered from pneumothorax due to prolonged air leakage, while 12 patients (16.9%) developed pneumonia and five (7.0%) required non-invasive or invasive ventilation. One patient was re-intubated due to septic shock. Cardiac complications occurred in 17 patients (23.9%), including atrial fibrillation and tachyarrhythmia which could be managed by electrolyte substitution and medication like beta-blockade or amiodarone. Impaired wound healing was observed in 5 patients (7.0%) and was effectively controlled by local measures. Leukopenia, hair loss or hepatic toxicity as known doxorubicin related side effects were not observed. Temporal renal insufficiency was observed in one patient as an undesirable side effect which did not show up in other patients with adequate diuresis. Severe complications with the need for reoperation (grade 3 complications) occurred in ten patients (14.1%). Three patients suffered from persistent chylothorax while six patients required surgical reintervention for closure of prolonged air leakage. Postoperative bleeding occurred in a patient with T4 sarcomatoid MPM with the need of surgical intervention. Thirty-day mortality was 1.4% due to one patient who died from acute pulmonary embolism on day 15 while 90-day mortality was 2.8% because of another patient who died on postoperative day 87 after severe pneumonia and sepsis which is comparable to recently published data of a meta-analysis concerning P/D without HITHOC with a perioperative mortality of 2% (15).
Impact of intervention on lung function
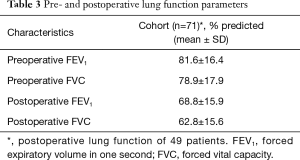
Mean preoperative FEV1 was 81.6%±16.4% predicted while postoperative FEV1 was 68.8%±15.9% predicted (both mean ± SD; P<0.0001). Mean FVC was 78.9%±17.9% predicted before the operation and 62.8%±15.6% predicted (both mean ± SD; P<0.0001) 3 to 6 months after surgery (Table 3). Postoperative lung function parameters from 49 patients were analyzed during follow-up.
Full table
Outcome
Fifty-five patients presented with epithelial, five with sarcomatoid and eleven patients with biphasic subtype. An overview of histologic subtypes and postoperative TNM classification according to the 8th edition of the AJCC Cancer Staging Manual is displayed in Table 1. Median overall survival for the entire cohort was 16.1 (range, 10.9–38.4) months (Figure 1A). As anticipated, the histologic tumor subtype significantly impacted survival (P=0.0012), which was 9.2 months (range, 7.5–14.1 months) for sarcomatoid (P=0.0006), 10.9 months (range, 6.9–20.5 months) for biphasic (P=0.0281), and 17.9 months (range, 12.6–48.4 months) for epithelioid tumour type (Figure 1B). We next examined the cohort of patients with epithelioid MPM according to the extent of resection, and found that patients with macroscopic complete resection had statistically significantly prolonged survival with 28.2 months (range, 14.2–71.6 months) compared to patients with macroscopic incomplete resection with 13.1 months (range, 8.8–14.7 months) (P<0.0001; Figure 1C). Unexpectedly, overall survival was not clearly depended on the postoperative TNM stage according to the 8th edition of the American Joint Committee on Cancer Staging Manual (16). Seven patients were classified as T1, 35 patients presented with a T2, and 27 patients with a T3 status. Only two patients presented with a T4 tumour. Median survival was 17.9 months (range, 14.1–34.4 months) for postoperative T1, 18.5 months (range, 10.7–46.2 months) for postoperative T2, 15.1 months (range, 11.9–18.0 months) for postoperative T3 and 8.3 months (range, 6.5–10.0 months) for postoperative T4 status. There was no significant difference in overall survival according to the T-status when comparing T1, T2 and T3 (Figure 2). Due to statistical concerns, two patients with T4 tumor were excluded from further analysis. Median overall survival was not significantly different concerning the mediastinal lymph node status (Figure 2B). Patients without mediastinal lymph node metastases (N0) showed no significant better overall survival compared to nodal positive status (N+). Ten patients (14.1%) did not undergo surgical mediastinal lymph node evaluation due to significant mediastinal tumor masses or due to lack of implementation at the beginning of our series and were classified Nx.
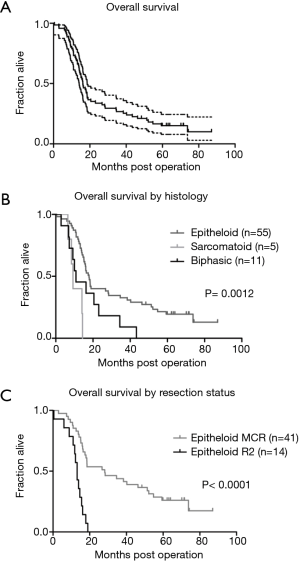
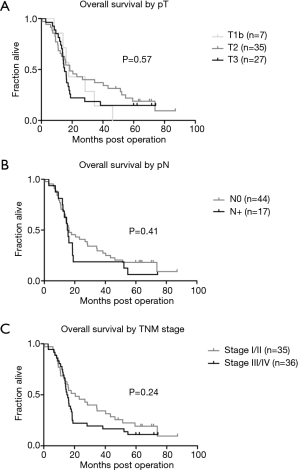
Gender, age, tumor location by side, body surface area or lung function data (FEV1) had no significant influence on overall survival using univariate analysis (data not shown). Multivariate analysis validated that histologic tumor subtype (epithelioid versus non-epithelioid) and completeness of resection were independent risk factors of overall survival (Table 4). Other variables like age, gender or extent of resection did not significantly impact survival as independent risk factors (Table 4).
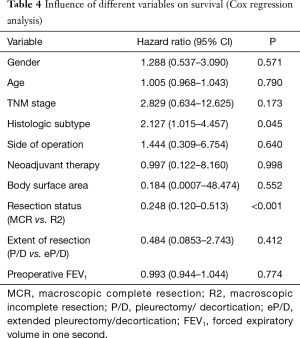
Full table
Discussion
Due to the long latency period of more than 30 years and its local tumour growth with late occurring symptoms, the diagnosis of MPM is often delayed and cancer is diagnosed in advanced stages. For resectable disease, a suitable treatment concept for older patients is required with the need to ensure a satisfying postoperative quality of life, macroscopic complete tumor resection and disease control should be accomplished. Effective cytoreductive surgery in combination with HITHOC is a promising treatment for localized pleural malignancies (6,8).
In this study, we analyze our experience with P/D and HITHOC in a multimodal treatment concept since 2009. We retrospectively analyzed the practicability and safety of the described treatment in our institution regarding morbidity and mortality. Due to the retrospective data collection, structured quality of life analysis could not be performed. Absence of mediastinal lymph node metastasis was present in more than 60% and macroscopic complete resection could be achieved in more than 70% of our patients which is superior to other cohorts reported (7,17). Macroscopic complete resection is one of the most important predictive factors for local recurrence and overall survival of MPM (6).
Due to the fact that no real “radical surgery” with microscopic complete resection margins is feasible in mesothelioma surgery, HITHOC represents a promising modality to treat the remaining microscopic tumor burden in the thoracic cavity (6,18). Intracavitary chemotherapy using direct cytotoxic agents combines the advantage of high local concentrations of the cytotoxic agent with limited systemic side effects. Hyperthermic application improves the penetration depth and consequently the efficacy of the agents (19).
The majority of chemotherapeutic agents in the treatment of MPM are platinum- and/ or anthracycline-based (20). Cisplatin has been the preferably used substance for HITHOC in the past because of its effectiveness during chemotherapy for systemic mesothelioma treatment (6,21). In this study, a combination with doxorubicin was used for HITHOC to enhance the effect of local cytotoxicity as opposed to other studies using cisplatin alone (18,22). Doxorubicin also belongs to the commonly used systemic chemotherapeutic agents for mesothelioma and has been analyzed in numerous studies for MPM treatment (20,23). Moreover, doxorubicin showed enhanced activity under hyperthermic conditions (22).
A systematic analysis of tissue penetration, systemic uptake and pharmacokinetics of cisplatin and doxorubicin during HITHOC was performed by van Ruth and colleagues. Maximum systemic concentration of doxorubicin (21 mg per liter) was about one tenth of the concentration after intravenous administration (24). Regarding the incidence of 24% cardiac events in our analysis, any potential association to doxorubicin due to its cardiotoxic effect after intravenous administration has to be considered. Therefore, de Bree and colleagues studied cardiotoxic side effects using cisplatin and doxorubicin for chemoperfusion. Apart from intermittent postoperative atrial fibrillation no significant cardiac adverse events occurred in the short- and medium-term postoperative course (25).
Increasing cisplatin dosage leads to a higher oncological effect as well as to multiple side effects. Due to this, the maximum tolerated cisplatin perfusate concentration is scheduled at 100 mg per liter (21). Our selected perfusate concentration of 40 mg per liter is based on extensive pharmacological test results which include potential local and systemic side effects as well as systemic drug concentrations (24,25). According to the current literature, this dosage can be used with good treatment response, tolerance and acceptable cardio- and nephrotoxic side effects (8,25).
In general, postoperative complications like renal insufficiency and atrial fibrillation are also observed after pleurectomy and decortication without intraoperative chemoperfusion. To date, no clinical study has shown an association of a higher complication rate after additional HITHOC compared to surgery alone (15).
Median operating time of 385 minutes is comparable to other studies although there is little information on this point in the existing literature (26).
Small preoperative biopsies or analysis of pleural fluid cannot sufficiently help to identify the precise histology of MPM. Ultrasound- or computed tomography-guided biopsies show a sensitivity of 77–87% while needle biopsies or pleural fluid analysis present with a low sensitivity of 7–47% (10,27,28). Furthermore, biphasic subtype of MPM is often not detected during video-assisted thoracoscopy due to the very small sample size even if multiple biopsies from different anatomic areas are taken (6,29). Therefore, some patients that were initially classified with an epithelioid subtype following thoracoscopy were later verified to be of biphasic histology in the final pathologic analysis following P/D. Because of the impact of the histologic subtype on prognosis, the survival rates of the different tumor entities were analysed separately. Patients with epithelioid tumor subtype had a significantly better survival than patients with other histological subtypes (6,9). The survival of patients suffering from sarcomatoid MPM was low in our cohort so that we do not consider surgery in this cohort of patients since 2014.
Although we report a high postoperative morbidity in our cohort, more than 40% of all complications were classified as minor complications and were successfully relieved by conservative treatment. Severe complications with the need for reoperation were due to persistent air leakage or chylothorax (30). In relation to the existing literature, our morbidity is reasonable. On average, a morbidity of 40 to 65% after cytoreductive surgery with or without HITHOC is reported (9,18,21). Thirty-day and 90-day mortality in our cohort match with the existing literature and underline that P/D is feasible in the treatment concept of MPM with an acceptable morbidity and mortality compared to EPP (2,9,15,30). Moreover, the median age in our cohort of 70 years shows that P/D can also be performed in older patients with comparable morbidity to improve overall survival and quality of life by absence of pain due to tumor infiltration into the chest wall and recurrent pleural effusion with dyspnea. Due to the lung sparing operating technique, age alone should not be an exclusion criterion per se for surgical mesothelioma treatment (9).
The major limitation of our study is the lack of a control group receiving pleurectomy and decortication without HITHOC. Therefore, a multicentre study would be helpful to analyse a larger cohort which might help to detect differences in survival among the TNM subgroups and between patients with or without neoadjuvant chemotherapy. In addition, tumor volume could be measured to evaluate possible associations with outcome parameters.
Conclusions
In conclusion, HITHOC after P/D seems to be a safe approach compared to the recent literature evaluating P/D alone for the treatment of selected patients with epithelial MPM. Macroscopic complete resection is an important prognostic factor. Morbidity and mortality for this operative multimodality approach is acceptable.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bibby AC, Tsim S, Kanellakis N, et al. Malignant pleural mesothelioma: an update on investigation, diagnosis and treatment. Eur Respir Rev 2016;25:472-86. [Crossref] [PubMed]
- Opitz I. Management of malignant pleural mesothelioma-The European experience. J Thorac Dis 2014;6 Suppl 2:S238-52. [PubMed]
- Taioli E, Wolf AS, Camacho-Rivera M, et al. Determinants of Survival in Malignant Pleural Mesothelioma: A Surveillance, Epidemiology, and End Results (SEER) Study of 14,228 Patients. PLoS One 2015;10:e0145039. [Crossref] [PubMed]
- Billé A, Krug LM, Woo KM, et al. Contemporary Analysis of Prognostic Factors in Patients with Unresectable Malignant Pleural Mesothelioma. J Thorac Oncol 2016;11:249-55. [Crossref] [PubMed]
- Friedberg JS, Simone CB 2nd, Culligan MJ, et al. Extended Pleurectomy-Decortication-Based Treatment for Advanced Stage Epithelial Mesothelioma Yielding a Median Survival of Nearly Three Years. Ann Thorac Surg 2017;103:912-9. [Crossref] [PubMed]
- Sugarbaker DJ, Gill RR, Yeap BY, et al. Hyperthermic intraoperative pleural cisplatin chemotherapy extends interval to recurrence and survival among low-risk patients with malignant pleural mesothelioma undergoing surgical macroscopic complete resection. J Thorac Cardiovasc Surg 2013;145:955-63. [Crossref] [PubMed]
- Lang-Lazdunski L, Bille A, Papa S, et al. Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine, prophylactic radiotherapy, and systemic chemotherapy in patients with malignant pleural mesothelioma: a 10-year experience. J Thorac Cardiovasc Surg 2015;149:558-65; discussion 565-6. [Crossref] [PubMed]
- Ried M, Potzger T, Braune N, et al. Cytoreductive surgery and hyperthermic intrathoracic chemotherapy perfusion for malignant pleural tumours: perioperative management and clinical experience. Eur J Cardiothorac Surg 2013;43:801-7. [Crossref] [PubMed]
- Williams T, Duraid H, Watson S, et al. Extended Pleurectomy and Decortication for Malignant Pleural Mesothelioma Is an Effective and Safe Cytoreductive Surgery in the Elderly. Ann Thorac Surg 2015;100:1868-74. [Crossref] [PubMed]
- Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010;35:479-95. [Crossref] [PubMed]
- Rice DC, Steliga MA, Stewart J, et al. Endoscopic ultrasound-guided fine needle aspiration for staging of malignant pleural mesothelioma. Ann Thorac Surg 2009;88:862-8; discussion 868-9. [Crossref] [PubMed]
- Nakas A, Waller D, Lau K, et al. The new case for cervical mediastinoscopy in selection for radical surgery for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2012;42:72-6; discussion 76. [Crossref] [PubMed]
- Cao C, Tian D, Park J, et al. A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer 2014;83:240-5. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- van Gerwen M, Wolf A, Liu B, et al. Short-term outcomes of pleurectomy decortication and extrapleural pneumonectomy in mesothelioma. J Surg Oncol 2018;118:1178-87. [Crossref] [PubMed]
- Nowak AK, Chansky K, Rice DC, et al. The IASLC Mesothelioma Staging Project: Proposals for Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Pleural Mesothelioma. J Thorac Oncol 2016;11:2089-99.
- Batirel HF, Metintas M, Caglar HB, et al. Adoption of pleurectomy and decortication for malignant mesothelioma leads to similar survival as extrapleural pneumonectomy. J Thorac Cardiovasc Surg 2016;151:478-84. [Crossref] [PubMed]
- Helm JH, Miura JT, Glenn JA, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: a systematic review and meta-analysis. Ann Surg Oncol 2015;22:1686-93. [Crossref] [PubMed]
- van Ruth S, Baas P, Haas RL, et al. Cytoreductive surgery combined with intraoperative hyperthermic intrathoracic chemotherapy for stage I malignant pleural mesothelioma. Ann Surg Oncol 2003;10:176-82. [Crossref] [PubMed]
- Steele JP, Klabatsa A. Chemotherapy options and new advances in malignant pleural mesothelioma. Ann Oncol 2005;16:345-51. [Crossref] [PubMed]
- Richards WG, Zellos L, Bueno R, et al. Phase I to II study of pleurectomy/decortication and intraoperative intracavitary hyperthermic cisplatin lavage for mesothelioma. J Clin Oncol 2006;24:1561-7. [Crossref] [PubMed]
- de Bree E, van Ruth S, Baas P, et al. Cytoreductive surgery and intraoperative hyperthermic intrathoracic chemotherapy in patients with malignant pleural mesothelioma or pleural metastases of thymoma. Chest 2002;121:480-7. [Crossref] [PubMed]
- Stathopoulos J, Antoniou D, Stathopoulos GP, et al. Mesothelioma: treatment and survival of a patient population and review of the literature. Anticancer Res 2005;25:3671-6. [PubMed]
- van Ruth S, van Tellingen O, Korse CM, et al. Pharmacokinetics of doxorubicin and cisplatin used in intraoperative hyperthermic intrathoracic chemotherapy after cytoreductive surgery for malignant pleural mesothelioma and pleural thymoma. Anticancer Drugs 2003;14:57-65. [Crossref] [PubMed]
- de Bree E, van Ruth S, Schotborgh CE, et al. Limited cardiotoxicity after extensive thoracic surgery and intraoperative hyperthermic intrathoracic chemotherapy with doxorubicin and cisplatin. Ann Surg Oncol 2007;14:3019-26. [Crossref] [PubMed]
- van Sandick JW, Kappers I, Baas P, et al. Surgical treatment in the management of malignant pleural mesothelioma: a single institution's experience. Ann Surg Oncol 2008;15:1757-64. [Crossref] [PubMed]
- Husain AN, Colby T, Ordonez N, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med 2013;137:647-67. [Crossref] [PubMed]
- Attanoos RL, Gibbs AR. The comparative accuracy of different pleural biopsy techniques in the diagnosis of malignant mesothelioma. Histopathology 2008;53:340-4. [Crossref] [PubMed]
- Bueno R, Reblando J, Glickman J, et al. Pleural biopsy: a reliable method for determining the diagnosis but not subtype in mesothelioma. Ann Thorac Surg 2004;78:1774-6. [Crossref] [PubMed]
- Taioli E, Wolf AS, Flores RM. Meta-analysis of survival after pleurectomy decortication versus extrapleural pneumonectomy in mesothelioma. Ann Thorac Surg 2015;99:472-80. [Crossref] [PubMed]


