
Can the robot overcome technical challenges of thoracoscopic bronchial anastomosis?
In patients with tumors of the right upper lobe encroaching upon the orifice of the lobar bronchus, right upper lobe sleeve resection is performed to avoid highly morbid right pneumonectomy while still achieving a negative resection margin. Although video-assisted and robotic lobar resections are quite common, most surgeons still use an open approach in patients who require a sleeve resection due to the additional complexity of the bronchial anastomosis.
In this issue, Huang et al. describe their approach to robotic sleeve right upper lobectomy. As they point out in their introduction, thoracoscopic sleeve lobectomy has been reported but not widely adopted due to the technical difficulty of thoracoscopic airway reconstruction. This suggests a promising application for the robot, where the surgeon can leverage increased wrist motion and maneuverability, as well as the magnified three-dimensional view the robot provides, to facilitate precise minimally-invasive suturing. In fact, many surgeons find the transition from open to robotic surgery more intuitive than that from open to video-assisted thoracic surgery, particularly when it comes to suturing.
Robotic sleeve resection was first reported by Schmid et al. in 2011 (1). Since then, there have been a number of case reports and case series describing variations on feasible techniques for robotic sleeve resection, as shown in Table 1 (2-6). For example, whereas Huang et al. use a method that includes a utility port, Cerfolio has described an experience with a totally portal technique (four robotic ports and one assistan.t port) (3). Although a totally portal approach has some advantages (use of insufflation, additional robot arm for surgeon-controlled retraction, smaller incisions), the use of a utility port could potentially reduce the risk of collisions between arms, allow a mechanism for dealing with catastrophic bleeding, and potentially provide an easier transition for surgeons at the earlier part of the learning curve for robotic lung resection (7,8). Other variables in technique described in prior publications include method of suturing for the anastomosis (interrupted, running, or combination), type of suture used (absorbable versus non-absorbable), and technique of cutting the bronchus (cautery versus scissors). These factors could be considered controversial, as they theoretically impact the risk of stenosis, suture line granuloma formation, and compromise of the blood supply at the anastomosis, respectively. One variable unique to Huang’s report is the concept of dissecting out and transecting the bronchus first, to create greater space and expedite the steps of the remainder of the lung resection. Although management of the pulmonary arteries and vein are generally no different than in the standard lobectomy, in a sleeve resection where the tumor is frequently centrally located, this strategy may be important in providing improved exposure and confirming the feasibility of the planned procedure by sending for frozen section analysis of the margins early on in the operation.
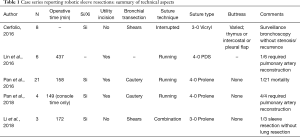
Full table
In addition to a meticulously performed bronchial anastomosis, critical basic principles that apply to all approaches to sleeve lobectomy include an anastomosis without tension and the use of tissue flaps to buttress tenuous reconstructions. Release maneuvers when there is concern for excessive anastomotic tension include mobilization of the trachea (via mediastinoscopy), transection of the inferior pulmonary ligament, and a hilar release (U-shaped pericardial incision just below the inferior pulmonary vein). These are all procedures that are easily incorporated into robotic thoracic operations. For high risk cases, the bronchial anastomosis can be buttressed with several options—pedicled intercostal muscle flap, pleural flap, pericardial fat pad, or omental flap. Although harvest of an intercostal muscle flap is easier in open surgery, pleural flaps and pericardial fat are readily available in robotic surgery.
Specific cases which may pose particular challenges that are difficult to address regardless of approach include bronchoplasties where there may be significant size mismatch or problems with airway alignment. In particular, lower lobe bronchoplasty may require rotation of the upper lobe bronchus to align the airway properly, and proximal airway resection can create a size mismatch that some authors deal with by telescoping the distal airway in to the larger proximal airway. Also, when the tumor involves both the bronchus and pulmonary artery, sleeve resection of both structures may be required. In select patients, this may be oncologically appropriate, but increased morbidity and mortality should be acknowledged, and judicious use of tissue flaps is needed to avoid broncho-vascular fistula. Reports of these combined bronchial and vascular resections suggest that the robotic approach is also possible in these cases (4,6).
As minimally invasive sleeve lobectomy becomes more common, it will be important going forward to assess whether there is any difference in the rate of short and long term complications. Pan et al. in their description of 21 cases list a 19% overall complication rate, including subcutaneous emphysema, cardiac arrhythmia, pneumonia, empyema, bronchial anastomosis bleeding, bronchial anastomosis leakage, and multiple organ failure, with a 30-day mortality rate of 4.8% (4). Only one report of 8 patients describes 6-month results (no complications) (3). However, clearly, longer term outcomes are needed to assess late complications such as anastomotic stricture and tumor recurrence between open, thoracoscopic, and robotic sleeve resections. This may be difficult to determine due to the relatively rare incidence of sleeve lobectomy.
Regarding postoperative recovery, we examined outcomes after robotic, thoracoscopic, and open lobectomies in the Nationwide Readmissions Database and found reduced readmission rates, mortality, and index hospitalization costs in minimally invasive compared to open surgery, and slightly higher costs in robotic versus thoracoscopic surgery, but otherwise similar outcomes between the two minimally invasive approaches (9). Others have reported lower conversation rate to open with robotic as opposed to thoracoscopic approach, which might be explained by the increased maneuverability and range of motion with the robot (10). As with other minimally invasive thoracic operations, it seems probable that oncologic results will be similar and perioperative outcomes improved with robotic sleeve resection (11). As robotic thoracic surgery gains momentum, we believe the advantages of the robot may be particularly relevant in successful minimally invasive sleeve lobectomy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Schmid T, Augustin F, Kainz G, et al. Hybrid video-assisted thoracic surgery-robotic minimally invasive right upper lobe sleeve lobectomy. Ann Thorac Surg 2011;91:1961-5. [Crossref] [PubMed]
- Li C, Zhou B, Han Y, et al. Robotic sleeve resection for pulmonary disease. World J Surg Oncol 2018;16:74. [Crossref] [PubMed]
- Cerfolio RJ. Robotic sleeve lobectomy: technical details and early results. J Thorac Dis 2016;8:S223-6. [PubMed]
- Pan X, Gu C, Yang J, et al. Robotic double-sleeve resection of lung cancer: technical aspects. Eur J Cardiothorac Surg 2018;54:183-4. [Crossref] [PubMed]
- Pan X, Gu C, Wang R, et al. Initial Experience of Robotic Sleeve Resection for Lung Cancer Patients. Ann Thorac Surg 2016;102:1892-7. [Crossref] [PubMed]
- Lin MW, Kuo SW, Yang SM, et al. Robotic-assisted thoracoscopic sleeve lobectomy for locally advanced lung cancer. J Thorac Dis 2016;8:1747-52. [Crossref] [PubMed]
- Ramadan OI, Wei B, Cerfolio RJ. Robotic surgery for lung resections-total port approach: advantages and disadvantages. J Vis Surg 2017;3:22. [Crossref] [PubMed]
- Abbas AE. New nomenclature for robotic-assisted thoracic surgery also gets rid of RATS. J Thorac Cardiovasc Surg 2017;154:1070-1. [Crossref] [PubMed]
- Bailey KL, Merchant N, Seo YJ, et al. Short-Term Readmissions After Open, Thoracoscopic, and Robotic Lobectomy for Lung Cancer Based on the Nationwide Readmissions Database. World J Surg 2019;43:1377-84. [Crossref] [PubMed]
- Reddy RM, Gorrepati ML, Oh DS, et al. Robotic-Assisted Versus Thoracoscopic Lobectomy Outcomes From High-Volume Thoracic Surgeons. Ann Thorac Surg 2018;106:902-8. [Crossref] [PubMed]
- Carlsson S, Jäderling F, Wallerstedt A, et al. Oncological and functional outcomes 1 year after radical prostatectomy for very-low-risk prostate cancer: results from the prospective LAPPRO trial. BJU Int 2016;118:205-12. [Crossref] [PubMed]


