
Current management and perspectives in the treatment of ventricular assist device-specific infections
Introduction
The number of patients on ventricular assist device (VAD) is annually growing. In Germany over approximately the last decade the number of VAD implantations increased from 462 to 1,027 (2017) (1). Contributing factors are the extent of indications for VAD therapy and the limited organ availability for heart transplantation. In this context, the number of patients awaiting heart transplantation in the Eurotransplant area increased between 2007 and 2017 from 959 to 1,141 (2). Thus, alternative therapies for treatment of heart failure are becoming increasingly necessary. Nowadays, new generation VADs allow patients to be bridged to transplantation for longer period. In the same time, new generation VADs allow a more acceptable quality of life for destination (DT) therapy patients (3-5). However, as a result of longer support period, a growing number of patients are exposed on the long run to VAD related complications such as infections. With an incidence rate of 19% in the first year, driveline infection can severely impact quality of life and limit life expectancy (6). Moreover, additional costs for frequent hospital readmissions and prolonged hospital stay are to be considered (7). Thus, beside a rigid protocol to prevent infections, an early recognition and treatment is of utmost importance once VAD-specific infections occur. Aim of this review is to report the modern diagnostic tools and treatments for the management of the VAD- specific infections.
Etiology, incidence, risk factors and classification of VAD infection
Bacteria are the most frequent cause of VAD infections. Specifically, staphylococcus aureus and epidermidis are responsible for up to 50% of the cases (8,9). Pseudomonas or other Gram-negative bacteria account for 32% of infected devices whereas fungal infections represent less than 10% of the infections (8,10). Identification of risk factors for the occurrence of the VAD-specific infections remains still controversial in the current literature. In a prospective study, dealing with 150 VAD patients, depression and elevated baseline serum creatinine were independent risk factors responsible for the occurrence of VAD-specific infections (8). In another study analyzing data of 2,006 left ventricular assist device (LVAD) recipients, Goldstein and colleagues found that younger age was the only independent risk factor (6).
Of note, patients with driveline infection had a significantly reduced survival on long term run than patients without driveline infection (6). This fact highlights once more the importance of prevention, prompt diagnosis and sufficient treatment of driveline infections. As reported by Koval et al. peak hazard for development of driveline infection is reached at 7.5 months after implantation (11). Prior publications classified VAD infection differently and each center has established its own criteria of definition (12). To this purpose a working formulation for standardization of definitions of VAD infections was published in 2017 by the International Society for Heart and Lung Transplantation (ISHLT) (12). In this document infections were classified into VAD-specific infections, VAD-related infections and non-VAD infections. VAD related infection included blood stream infection endocarditis, mediastinitis and sternal wound infections, whereas VAD-specific infections have been classified as infections specifically involving the LVAD-components including pump housing, driveline and inflow/outflow graft (12). Moreover, VAD-specific infections have been appropriately subdivided into proven, probable and possible, based on the microbiological and clinical findings (12). The latter two definitions (probable and possible) are based of clinical assessment alone. On the other hand, through a multi-assessment process, which includes clinical, microbiological and radiological assessment VAD-specific infections can be classified as “proven”. Regarding the extent of the infection, VAD specific-infections have been classified in superficial and deep infections depending on internal extent of infection (12).
Diagnosis of VAD-specific infections
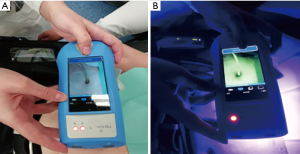
As above-mentioned, clinical, microbiological and radiological assessments are the first approaches for diagnosis of infection. However, before becoming clinically evident, an earlier detection may help physician start an early treatment in order to prevent further extent of infection to the internal components. In this setting we are conducting a prospective study using fluorescence-imaging device for the early identification of infection (Figure 1). This is a real-time camera used to visualize the surface bacterial concentration (13). It uses violet light illumination and allows locating high bacteria concentration. Through this device swabs can be guided to areas of high concentration of bacteria as indicated by fluorescence (Figure 2). Ongoing analyses are aiming to ascertain the sensibility and specificity of this device in this setting.
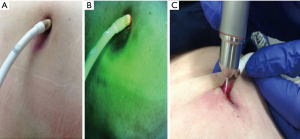
Concerning to the identification of deep driveline infection the clinical inspection of driveline can approximately help physician recognize the internal extent of infection. However clinical inspection alone can underestimate the infection depth. In fact, some infections can involve the internal components without signs of superficial driveline infection. This can typically occur in patients where infection is locally treated without reaching the internal parts of the cable. In those cases, no signs of infection can be superficially observed and swabs remain negative despite patients continuing to have a deep VAD-infection. In this situation further radiological or nuclear medicine examinations can help clinicians to establish diagnosis. In 2015 we retrospectively reviewed our experience with the use of fluorine 18-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) for detection of VAD-specific infections. Including a total of 40 PET/CT examinations we found a specificity and sensitivity of 100% and 80% respectively. Moreover, those findings contributed to changing the clinical management in over 80% of the patients. Those changes included a more aggressive and prolonged antibiotic therapy, urgent transplantation, tissue debridement around the driveline, and local treatment of other foci in the true positive cases. Conversely, in true negative cases, interruption of antibiotic therapy with early discharge could be undertaken (14).
In line with our results Bernhardt et al. including a cohort of 27 PET/CT examinations reported high sensitivity (87.5%) and specificity (100%) as well (15). In a further study including a higher number of examinations (n=61), we could confirm the high specificity and sensibility for the superficial and deep driveline infections. However, we had to reconsider our enthusiastic approach regarding the detection of pump housing infection (16). In fact, the high diagnostic power of PET/CT in detecting driveline infection could not be confirmed for pump housing infections (P=0.33) (17). In fact, the high quantity of artefacts generated by the pump housing, the physiological tracer uptake into the adjacent left ventricular myocardium and possible involvement in the infection of inner surface of the pump housing invisible to the PET/CT can mislead data interpretation.
Besides clinical, laboratory and microbiological findings we rely also on PET/CT assessments taking as reference value a cut-off SUVmax of 4 along the driveline for the diagnosis of infection. Simultaneously we call for caution when interpreting the PET/CT quantitative assessments for the pump housing. In those cases, diagnosis of pump housing infection must be based especially on clinical data whereas PET assessments must be preponderantly based on visual interpretation (not quantitative ones), such as the presence of reactive mediastinal/axillary lymph nodes and comparison of different PET/CT scans performed at follow-up (17).
Treatment VAD-specific infections
The 2017 “ISHLT consensus document for prevention and management strategies for MCS infection” has been published with the aim to provide clear suggestions for LVAD-specific and related infections treatment (18). Concerning the superficial driveline infections, this document has recommended the use of antibiotics for a minimum of 2 weeks. In our center in order to reduce adverse events and minimize the development of resistances we optimize antibiotic use according to the antibiotic stewardship regardless of the treatment duration (19). Moreover, in order to prevent relapse of infection since 2018 we routinely use hypochlorite rinsing solutions and cold plasma therapy for the local treatment. The latter can be more specifically applied to highest bacteria concentration around the piercing site through the real time guidance of immunofluorescence (Figure 3). The therapeutic principles of hypochlorite rinsing solutions and cold plasma treatment reside in the release of reactive oxygen and nitrogen species to destroy bacteria (20,21). Noteworthy, the rinsing solution can superficially wash out the destroyed bacteria, cold plasma through its gaseous condition can deeply penetrate the tissue in order to prevent further deep colonization (Figure 2).

Concerning the treatment of deep driveline infection, the ISHLT consensus document has suggested a surgical debridement with or without wound vacuum-assisted closure (VAC) along with the antibiotic treatment. For more aggressive infection including either pump/cannula infection, relapsed infections or for uncontrolled infection a more drastic treatment such as LVAD removal by means of heart transplantation in bridge-to-transplant (BTT) patients and suppression antibiotic treatment or device replacement in BTT patients has been appropriately recommended. However, pump exchange in DT therapy patients with chronic infection of LVAD may be notably challenging considering the perioperative mortality risk. In the same way prolonged waiting time due to the ongoing organ shortage before heart transplant can compromise the benefit of transplantation in BTT patients. In this setting a new treatment strategy has been developed in our center. After surgical debridement the wound is treated with hypochlorite rinsing solutions and cold plasma as above-mentioned. Additionally, we applied a carbon cloth underneath the foam of the VAC system in order to maintain the superficial infected field sterile during the whole treatment time (Figure 4). This modified VAC treatment contributes to immobilize residual bacteria and improving the wound healing by binding macromolecules, such as MMP-2 and MMP-9 in the wound area, which inhibit healing in chronic wounds (22,23). Through this combination treatment we could achieve a complete healing of 6 out of 9 patients with a relapsed LVAD-infection whereas the remaining three patients were discharged with well-controlled infection.

Conclusions
Occurrence of VAD-specific infections is a serious complication that can jeopardize on the long run the effectiveness of VAD therapy. In the light of ongoing shortage of organ available for heart transplantation there will be in the next future an increasing necessity of conservative strategies. A multidisciplinary approach including cardiac surgeons, cardiologist, ID consultant and nuclear medicine physicians should be the first step for the diagnosis and treatment of VAD-specific infections. Early detection of infections and consequently early treatment with innovative strategies may help physicians to improve outcomes.
Acknowledgments
None.
Footnote
Conflicts of Interest: H Rotering is consultant for the following companies: Serag Wissner, Smith@Nephew and Adtec. The other authors have no conflicts of interest to declare.
References
- Beckmann A, Meyer R, Lewandowski J, et al. German Heart Surgery Report 2017: The Annual Updated Registry of the German Society for Thoracic and Cardiovascular Surgery. Thorac Cardiovasc Surg 2018;66:608-21. [Crossref] [PubMed]
- Available online: https://www.eurotransplant.org/cms/index.php?page=annual_reports
- Dell'Aquila AM, Schneider SR, Schlarb D, et al. Initial clinical experience with the HeartWare left ventricular assist system: a single-center report. Ann Thorac Surg 2013;95:170-7. [Crossref] [PubMed]
- Mehra MR, Naka Y, Uriel N, et al. MOMENTUM 3 Investigators. A fully magnetically levitated circulatory pump for advanced heart failure. N Engl J Med 2017;376:440-50. [Crossref] [PubMed]
- Uriel N, Colombo PC, Cleveland JC, et al. Hemocompatibility-Related Outcomes in the MOMENTUM 3 Trial at 6 Months: A Randomized Controlled Study of a Fully Magnetically Levitated Pump in Advanced Heart Failure. Circulation 2017;135:2003-12. [Crossref] [PubMed]
- Goldstein DJ, Naffel D, Holman W, et al. Continuous-flow devices and percutaneous site infections: clinical outcomes J Heart Lung Transplant 2012;31:1151-7. [Crossref] [PubMed]
- Akhter SA, Badami A, Murray M, et al. Hospital Readmissions After Continuous-Flow Left Ventricular Assist Device Implantation: Incidence, Causes, and Cost Analysis. Ann Thorac Surg 2015;100:884-9. [Crossref] [PubMed]
- Gordon RJ, Weinberg AD, Pagani FD, et al. Prospective, multicenter study of ventricular assist device infections.; Ventricular Assist Device Infection Study Group. Circulation 2013;127:691-702. [Crossref] [PubMed]
- Nienaber JJ, Kusne S, Riaz T, et al. Clinical manifestations and management of left ventricular assist device-associated infections. Clin Infect Dis 2013;57:1438-48. [Crossref] [PubMed]
- Gordon SM, Schmitt SK, Jacobs M, et al. Nosocomial bloodstream infections in patients with implantable left ventricular assist devices. Ann Thorac Surg 2001;72:725-30. [Crossref] [PubMed]
- Koval CE, Thuita L, Moazami N, et al. Evolution and impact of drive-line infection in a large cohort of continuous-flow ventricular assist device recipients. J Heart Lung Transplant 2014;33:1164-72. [Crossref] [PubMed]
- Hannan MM, Husain S, Mattner F, et al. Working formulation for the standardization of definitions of infections in patients using ventricular assist devices, International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2011;30:375-84. [Crossref] [PubMed]
- Blumenthal E, Jeffery SLA. The Use of the MolecuLight i:X in Managing Burns: A Pilot Study. J Burn Care Res 2018;39:154-61. [PubMed]
- Dell'Aquila AM, Mastrobuoni S, Alles S, et al. Contributory Role of Fluorine 18-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in the Diagnosis and Clinical Management of Infections in Patients Supported With a Continuous-Flow Left Ventricular Assist Device. Ann Thorac Surg 2016;101:87-94; discussion 94. [Crossref] [PubMed]
- Bernhardt AM, Pamirsad MA, Brand C, et al. The value of fluorine-18 deoxyglucose positron emission tomography scans in patients with ventricular assist device specific infections. Eur J Cardiothorac Surg 2017;51:1072-7. [Crossref] [PubMed]
- Dell'Aquila AM, Avramovic N, Mastrobuoni S, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography for improving diagnosis of infection in patients on CF-LVAD: longing for more 'insights'. Eur Heart J Cardiovasc Imaging 2018;19:532-43. [Crossref] [PubMed]
- Dell'Aquila AM, Sindermann JR. 18F-FDG positron emission tomography/computed tomography for diagnosis of pump housing infections in patients on left ventricular assist devices: should we contain our initial enthusiasm? Eur J Cardiothorac Surg 2018;53:892-6. [Crossref] [PubMed]
- Kusne S, Mooney M, Danziger-Isakov L, et al. An ISHLT consensus document for prevention and management strategies for mechanical circulatory support infection. J Heart Lung Transplant 2017;36:1137-53. [Crossref] [PubMed]
- Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016;62:e51-77. [Crossref] [PubMed]
- Chambers AC, Leaper DJ. Role of oxygen in wound healing: a review of evidence. J Wound Care 2011;20:160-4. [Crossref] [PubMed]
- Dahlgren C, Karlsson A. Respiratory burst in human neutrophils J Immunol Methods 1999;232:3-14. [Crossref] [PubMed]
- Zorflex® Carbon Cloth Dressings. Available online: http://novagranproducts.com/zorflex-carbon-cloth-dressings/
- Kramer A, Dissemond J, Kim S, et al. Consensus on Wound Antisepsis: Update 2018. Skin Pharmacol Physiol 2018;31:28-58. [Crossref] [PubMed]


