
Serum tumor marker levels at the development of intracranial metastasis in patients with lung or breast cancer
Introduction
Serum tumor markers, including carcinoembryonic antigen (CEA), pro-gastrin-releasing peptide (ProGRP), neuron-specific enolase (NSE), and cancer antigen 15-3 (CA15-3), are widely used as prognostic factors or factors to predict the therapeutic response in patients with lung or breast cancer. High levels of these markers in lung or breast cancer indicate poor prognosis and therapeutic response (1-4). Lung and breast cancers often cause intracranial metastasis (IM), including in the central nervous system (CNS). Elevated serum CEA, ProGRP, and NSE levels have been reported as predictive factors for CNS metastasis development (5-8). Although these reports measured the serum tumor marker levels before CNS metastasis development, such as during the pretreatment of a primary tumor, serum tumor markers during CNS metastasis development have not been examined. A previous study on the prevalence of IM according to the primary tumor found that lung and breast cancers were the two major primary tumors associated with the development of IM (9). We encountered patients with lung or breast cancer developing IM who showed no increase in the serum tumor marker levels. Thus, this is the first study to examine patients with IM without elevated serum tumor marker levels using brain magnetic resonance imaging (MRI) findings.
Methods
Patients
We studied 53 patients with lung or breast cancer as the primary tumor with newly detected IM by enhanced brain MRI between January 2013 and December 2018. The age when IM was detected by MRI was defined as the age at IM. The present study was a retrospective study approved by the Institutional Review Board of Nihon University School of Medicine, and informed consent was obtained from all patients.
Detection of IM
IM was evaluated using gadolinium-enhanced T1-weighted, non-enhanced T1- and T2-weighted, fluid-attenuated inversion recovery (FLAIR), and diffusion-weighted MRI images. IM was classified into three types: (I) parenchymal metastasis (PM), in which nodular enhancement is detected in the brain parenchyma; (II) leptomeningeal metastasis (LM), in which linear, nodular, or miliary enhancement is detected on the brain surface such as the gyri and sulci, tentorium, or leptomeningeal of the ventricular ependymal surfaces (10-12); and (III) dural metastasis (DM), in which diffuse or nodular enhancement is detected on the thickening of the dura mater (12,13). When clarifying whether nodular enhancement was observed in the parenchymal or brain surface using enhanced T1-weighted images was difficult, PM was differentiated from LM by referring to the FLAIR images and T2-weighted coronal and sagittal images. Unlike in LM, in DM, the gyri and sulci are not sequentially enhanced; therefore, it was determined on the basis of sparing the subarachnoid space (12). The number of days from the initial treatment of a primary tumor to the detection of IM by MRI was defined as the time to IM development.
Extracranial metastatic site at IM
The number of extracranial metastatic sites was evaluated by performing chest, abdominal, and pelvic computed tomography (CT) immediately after the detection of IM using MRI and compared with the number of extracranial metastatic sites identified during the initial treatment. Each organ, lymphangitic spread, bone, pleura, and soft tissue was considered an extracranial metastatic site. For example, only increased numbers of liver metastases were defined as no increase in the number of sites.
Measurement of serum tumor marker levels
CEA is a serum tumor marker in non-small cell lung cancer (NSCLC). Serum CEA level was measured using an electrochemiluminescence immunoassay (ECLIA) with the Elecsys® CEAII kit (Roche Diagnostics, Rotkreuz, Switzerland) or chemiluminescent immunoassay with the CEA Abbott® kit (Abbott, IL, USA). ProGRP and NSE are tumor markers of small cell lung cancer (SCLC). Plasma ProGRP level was measured using a chemiluminescent enzyme immunoassay (CLEIA) with the Lumipulse Presto® ProGRP kit (Fujirebio, Tokyo, Japan), and serum NSE level was measured using ECLIA with the Elecsys® NSE kit (Roche Diagnostics, Rotkreuz, Switzerland). CA15-3 is a serum tumor marker of breast cancer. The serum CA15-3 level was measured using CLEIA with the Lumipulse Presto® CA15-3 kit (Fujirebio, Tokyo, Japan). The upper limits of the normal range were as follows: CEA, 5.0 ng/mL; ProGRP, 81 pg/mL; NSE, 16.3 ng/mL; and CA15-3, 30 U/mL. The serum tumor marker level on the day of measurement immediately shortest before or after IM detection using MRI was considered at the IM level. A level within the normal limit was defined as no increase.
Statistical analyses
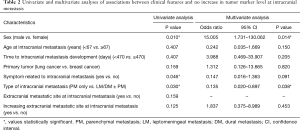
SPSS ver. 21.0 (IBM, Armonk NY, USA) was used for the statistical analyses. To analyze of associations between clinical features and no increase in tumor marker level at IM, univariate and multivariate analyses were performed using Pearson’s χ2 test and logistic regression analysis via the forced entry procedure, respectively, which were assessed based on the following patient characteristics and variables: sex, age at IM (< vs. ≥ median), time to IM development (< vs. median), primary tumor (lung vs. breast), symptoms related to IM (yes vs. no), type of IM (PM only vs. LM/DM ± PM), and increased extracranial metastatic sites at IM (yes vs. no). A P value of <0.05 was considered statistically significant.
Results
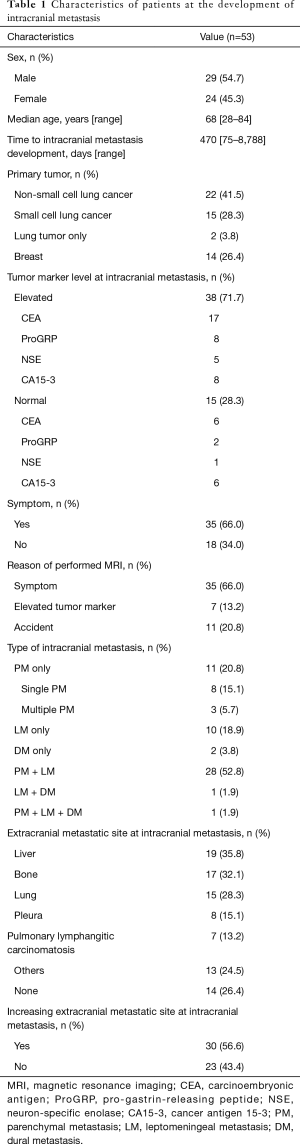
Table 1 lists the characteristics of the patients in this study. Of the 53 patients analyzed in this study, 29 (54.7%) were men and 24 (45.3%) were women. The median age at IM detection was 68 years (range, 28–84 years), and the median time to IM development was 470 days (range, 75–8,788 days). Regarding the primary tumor, 39 (73.6%) patients had lung cancer (19 with adenocarcinoma, 1 with adenosquamous cell carcinoma, 2 with squamous cell carcinoma, 15 with small cell carcinoma and 2 patients with histological type unknown) and 14 (26.4%) had breast cancer. Among the two patients with unknown histological type, one was classified as having NSCLC and evaluated using CEA. Since a histological test was not performed on the other patient, the patient was evaluated with ProGRP due to the high ProGRP levels in the patient before the initial treatment.
Full table
Among the total 53 patients, 15 (28.3%) showed no increase in the serum tumor marker levels at IM detection. Among the 23 NSCLC patients, 6 (26.1%) showed no increase in CEA level; among the 12 SCLC patients, 2 (16.7%) showed no increase in ProGRP level; and among the 4 patients with SCLC, 1 (25.0%) showed no increase in NSE level. Among the 14 patients with breast cancer, 6 (42.9%) showed no increase in CA15-3 level. Symptoms related to IM were observed in 35 patients (66.0%).
Regarding the cause of performing MRI that detected IM, 7 patients (13.2%) with or without symptoms underwent MRI due to elevated serum tumor marker levels, and 11 (20.8%) underwent MRI incidentally. A total of 11 patients were classified as having PM only (20.8%) and 8 patients (15.1%) had single PM; 10 were classified as LM only (18.9%); 2 were classified as DM only (3.8%); 28 were classified as PM + LM (52.8%); 1 was categorized as LM + DM (1.9%); and 1 was classified with PM + LM + DM (1.9%). At detection of IM, 14 patients (26.4%) had no extracranial metastatic site. In 30 patients (56.7%), the number of extracranial metastatic sites at IM was increased compared with the number at initial examination.
According to the univariate and multivariate analysis results for associations between clinical features and no increase in serum tumor marker level at IM was noted in Table 2. In the χ2 test, the patients with PM only significantly correlated with no increasing tumor marker level compared with other IM types (P=0.030). Moreover, female patients and patients with symptoms also significantly correlated with no increasing tumor marker level (P=0.010 and 0.046, respectively). No significant differences were observed in other variables in the univariate analysis. In the logistic regression analysis, the patients with PM only significantly correlated with no increasing tumor marker level compared with other IM types (P=0.038) (Figure 1). In addition, female patients also significantly correlated with no increasing tumor marker level compared with male patients (P=0.014). No significant differences were observed in other variables in the multivariate analysis.
Full table
Discussion
Previous studies reported that the IM incidence rates after the initial treatment of patients with NSCLC, SCLC, and breast cancer without metastasis at the initial examination were 11.6–37% (5,8), 26.8–38% (7,14), and 3.4–28.7% (15,16), respectively. Over the past decade, the median time interval from the diagnosis of a primary tumor to IM development has been prolonged from 3 to 8 months (1983–1989 vs. 2005–2009) (9). In our patient group, which was analyzed starting in 2013, IM was detected using MRI and the time to IM development was prolonged to 470 days. This increase may be because of the progress in the systemic chemotherapy regimen used until IM development. A longer interval between the diagnosis of a primary tumor and IM development has been associated with increased survival, and a longer period between the occurrence of CNS symptoms and the start of treatment results in a poor therapeutic effect (17,18). Thus, timely diagnosis of IM development is important. Performing enhanced brain MRI periodically is necessary for early detection of IM; however, the deposition of gadolinium in the brain is currently controversial (19). As 58–84% of IM patients have some symptoms, such as headache, seizure, visual disturbance, or neurologic function disorders (20-22), MRI is often performed at the onset of symptoms. No previous study reported on the cause of performing MRI that detected IM after treating the primary tumor. In the present study, 66% of patients underwent MRI because of the onset of symptoms. In our patient group, 13.2% of patients without symptoms underwent MRI because of elevated serum tumor marker levels, which played a role in the early detection of IM. However, IM was detected in 28.3% of patients without elevated serum tumor marker levels. Thus, this study is the first to assess the types of patients with IM that did not show elevated serum tumor marker levels.
Many reports have been published on the relationship between serum tumor marker levels before initial treatment and IM development. Patients with lung adenocarcinoma and high serum CEA levels before initial treatment have been reported to be at a high risk for IM development (5,6). Patients with NSCLC and high serum NSE levels before the initial treatment have also been reported to be at a high risk for IM development (8). In another study, IM patients with NSCLC and distant metastasis before the initial treatment showed high serum CEA levels (23). In the report of serum tumor markers at the first recurrence, breast cancer patients showed higher serum CA15-3 levels in organ metastasis than in bone metastasis (24). Recently, IM cases diagnosed using MRI have been classified into subtypes, such as PM, LM, and DM, and individual treatment approaches for these subtypes, such as chemotherapy and targeted therapy, have been developed (25-27). Our patients were classified as having PM, LM, or DM based on the MRI findings, and the relationship with serum tumor marker levels was assessed. We found that the patients with PM only significantly correlated with no increasing tumor marker level compared with other IM types. This may be because the decrease in the blood-cerebrospinal fluid (CSF) barrier in LM. The decrease in the blood-CSF barrier in LM allows cytotoxic agents to easily reach the brain surface (28,29). This decrease in the CSF barrier provides easy access for tumor markers produced in LM tumors to flow into the CSF and then through the bloodstream. Therefore, in LM, the tumor marker levels in the serum may tend to increase more easily compared with those in cases with only PM. A previous study reported that ventricular CSF CEA levels of LM from a primary tumor, including lung and breast cancers, are higher when patients have hydrocephalus as a complication (30). The authors suggested that light particles such as CEA could cause backflow to the ventricle due to CSF congestion. Owing to this backflow, tumor markers produced in LM tumor would flow from the ventricle and then to the bloodstream, which may thus increase tumor marker levels in the serum more in LM than in PM only. However, CSF CEA level was also not correlated with the serum CEA level in the LM in breast cancer (31). Thus, the reasons for our results are currently unclear. We previously reported that the prognosis in patients with LM only was better than patients with other IM types (20). These results could indicate that patients with PM only did not show elevated serum tumor marker levels and thus IM was not detected at the early stage, which may be associated with the poor prognosis.
One limitation of this study was that the CSF level was not evaluated. Recent studies have examined the correlation between serum tumor marker levels and peripheral blood circulating tumor cells (CTC) in lung and breast cancers. For example, patients with lung cancer and with elevated serum NSE levels showed increased CTC counts (32,33). Measurement of CSF tumor marker levels and peripheral blood CTC counts in IM patients may help clarify the pathological conditions of patients with PM only among patients with IM without elevated serum tumor marker level in future studies.
Conclusions
In our patient group, 28.3% (15 patients) showed no increase in the serum tumor marker levels at IM detection. This notable percentage of patients with IM and without elevated tumor markers suggests a need to monitor lung and breast cancer patients or identify new indicators for IM and the need to distinguish the mechanism of PM from other IM subtypes in relation with the markers.
Acknowledgments
We thank Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The present study was a retrospective study approved by the Institutional Review Board of Nihon University School of Medicine (No. RK-190212-8), and informed consent was obtained from all patients.
References
- Hotta K, Segawa Y, Takigawa N, et al. Evaluation of the relationship between serum carcinoembryonic antigen level and treatment outcome in surgically resected clinical-stage I patients with non-small-cell lung cancer. Anticancer Res 2000;20:2177-80. [PubMed]
- Arrieta O, Saavedra-Perez D, Kuri R, et al. Brain metastasis development and poor survival associated with carcinoembryonic antigen (CEA) level in advanced non-small cell lung cancer: a prospective analysis. BMC Cancer 2009;9:119. [Crossref] [PubMed]
- Huang Z, Xu D, Zhang F, et al. Pro-gastrin-releasing peptide and neuron-specific enolase: useful predictors of response to chemotherapy and survival in patients with small cell lung cancer. Clin Transl Oncol 2016;18:1019-25. [Crossref] [PubMed]
- Shao Y, Sun X, He Y, et al. Elevated Levels of Serum Tumor Markers CEA and CA15-3 Are Prognostic Parameters for Different Molecular Subtypes of Breast Cancer. PLoS One 2015;10:e0133830. [Crossref] [PubMed]
- Horinouchi H, Sekine I, Sumi M, et al. Brain metastases after definitive concurrent chemoradiotherapy in patients with stage III lung adenocarcinoma: carcinoembryonic antigen as a potential predictive factor. Cancer Sci 2012;103:756-9. [Crossref] [PubMed]
- Wang W, Bian C, Xia D, et al. Combining Carcinoembryonic Antigen and Platelet to Lymphocyte Ratio to Predict Brain Metastasis of Resected Lung Adenocarcinoma Patients. Biomed Res Int 2017;2017:8076384. [PubMed]
- Yonemori K, Sumi M, Fujimoto N, et al. Pro-gastrin-releasing peptide as a factor predicting the incidence of brain metastasis in patients with small cell lung carcinoma with limited disease receiving prophylactic cranial irradiation. Cancer 2005;104:811-6. [Crossref] [PubMed]
- Ji Z, Bi N, Wang J, et al. Risk factors for brain metastases in locally advanced non-small cell lung cancer with definitive chest radiation. Int J Radiat Oncol Biol Phys 2014;89:330-7. [Crossref] [PubMed]
- Nieder C, Spanne O, Mehta MP, et al. Presentation, patterns of care, and survival in patients with brain metastases: what has changed in the last 20 years? Cancer 2011;117:2505-12. [Crossref] [PubMed]
- Collie DA, Brush JP, Lammie GA, et al. Imaging features of leptomeningeal metastases. Clin Radiol 1999;54:765-71. [Crossref] [PubMed]
- Gleissner B, Chamberlain MC. Neoplastic meningitis. Lancet Neurol 2006;5:443-52. [Crossref] [PubMed]
- Barajas RF Jr, Cha S. Imaging diagnosis of brain metastasis. Prog Neurol Surg 2012;25:55-73. [Crossref] [PubMed]
- Tyrrell RL 2nd, Bundschuh CV, Modic MT. Dural carcinomatosis: MR demonstration. J Comput Assist Tomogr 1987;11:329-32. [Crossref] [PubMed]
- Gregor A, Cull A, Stephens RJ, et al. Prophylactic cranial irradiation is indicated following complete response to induction therapy in small cell lung cancer: results of a multicentre randomised trial. United Kingdom Coordinating Committee for Cancer Research (UKCCCR) and the European Organization for Research and Treatment of Cancer (EORTC). Eur J Cancer 1997;33:1752-8. [Crossref] [PubMed]
- Sihto H, Lundin J, Lundin M, et al. Breast cancer biological subtypes and protein expression predict for the preferential distant metastasis sites: a nationwide cohort study. Breast Cancer Res 2011;13:R87. [Crossref] [PubMed]
- Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol 2010;28:3271-7. [Crossref] [PubMed]
- Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997;37:745-51. [Crossref] [PubMed]
- Yap HY, Yap BS, Rasmussen S, et al. Treatment for meningeal carcinomatosis in breast cancer. Cancer 1982;50:219-22. [Crossref] [PubMed]
- Kanda T, Nakai Y, Oba H, et al. Gadolinium deposition in the brain. Magn Reson Imaging 2016;34:1346-50. [Crossref] [PubMed]
- Sakaguchi M, Maebayashi T, Aizawa T, et al. Patient outcomes of whole brain radiotherapy for brain metastases versus leptomeningeal metastases: A retrospective study. Asia Pac J Clin Oncol 2017;13:e449-57. [Crossref] [PubMed]
- Oechsle K, Lange-Brock V, Kruell A, et al. Prognostic factors and treatment options in patients with leptomeningeal metastases of different primary tumors: a retrospective analysis. J Cancer Res Clin Oncol 2010;136:1729-35. [Crossref] [PubMed]
- Laigle-Donadey F, Taillibert S, Mokhtari K, et al. Dural metastases. J Neurooncol 2005;75:57-61. [Crossref] [PubMed]
- Lee DS, Kim YS, Jung SL, et al. The relevance of serum carcinoembryonic antigen as an indicator of brain metastasis detection in advanced non-small cell lung cancer. Tumour Biol 2012;33:1065-73. [Crossref] [PubMed]
- Berruti A, Tampellini M, Torta M, et al. Prognostic value in predicting overall survival of two mucinous markers: CA 15-3 and CA 125 in breast cancer patients at first relapse of disease. Eur J Cancer 1994;30A:2082-4. [Crossref] [PubMed]
- Tabouret E, Chinot O, Metellus P, et al. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res 2012;32:4655-62. [PubMed]
- Wang N, Bertalan MS, Brastianos PK. Leptomeningeal metastasis from systemic cancer: Review and update on management. Cancer 2018;124:21-35. [Crossref] [PubMed]
- Heo MH, Cho YJ, Kim HK, et al. Isolated pachymeningeal metastasis from breast cancer: Clinical features and prognostic factors. Breast 2017;35:109-14. [Crossref] [PubMed]
- Ushio Y, Shimizu K, Aragaki Y, et al. Alteration of blood-CSF barrier by tumor invasion into the meninges. J Neurosurg 1981;55:445-9. [Crossref] [PubMed]
- Siegal T, Sandbank U, Gabizon A, et al. Alteration of blood-brain-CSF barrier in experimental meningeal carcinomatosis. A morphologic and adriamycin-penetration study. J Neurooncol 1987;4:233-42. [Crossref] [PubMed]
- Shim Y, Gwak HS, Kim S, et al. Retrospective Analysis of Cerebrospinal Fluid Profiles in 228 Patients with Leptomeningeal Carcinomatosis: Differences According to the Sampling Site, Symptoms, and Systemic Factors. J Korean Neurosurg Soc 2016;59:570-6. [Crossref] [PubMed]
- Yap BS, Yap HY, Fritsche HA, et al. CSF carcinoembryonic antigen in meningeal carcinomatosis from breast cancer. JAMA 1980;244:1601-3. [Crossref] [PubMed]
- Wang X, Ma K, Yang Z, et al. Systematic Correlation Analyses of Circulating Tumor Cells with Clinical Variables and Tumor Markers in Lung Cancer Patients. J Cancer 2017;8:3099-104. [Crossref] [PubMed]
- Bidard FC, Hajage D, Bachelot T, et al. Assessment of circulating tumor cells and serum markers for progression-free survival prediction in metastatic breast cancer: a prospective observational study. Breast Cancer Res 2012;14:R29. [Crossref] [PubMed]


