
Decoding left bundle branch block: insights into the future of his-purkinje conduction system pacing
His bundle pacing (HBP) has the unique ability to deliver and replicate normal infra-nodal conduction (1). It has therefore been used in variety of pacing indications including pacing for bradycardia pacing and in patients undergoing atrioventricular (AV) node ablation (2,3). Furthermore, in some patients who have underlying bundle branch block (BBB), HBP has been able to narrow the QRS and reverse the conduction abnormality (4). This has enabled HBP to be studied in patients requiring biventricular pacing (BVP) for cardiac resynchronisation therapy (CRT) including those with failed left ventricular (coronary venous) lead placements or those who have not responded to BVP (5,6). Outcomes in general, have been positive with significant success in this group albeit in small non-randomised studies.
The mechanism of how HBP reverses BBB for long had been based on a key study by OS Narula which developed the concept of longitudinal dissociation with specific fibres within the His bundle committed to the left bundle with asynchronous conduction leading to left bundle branch block (LBBB) pattern (4). Localised lesions within the His bundle can therefore cause LBBB, and by pacing near or at a slightly more distal location within the His bundle can overcome the LBBB, and normalize conduction. Technical and patient related factors prevent HBP in reversing LBBB in many individuals. The success rates range from 75–90%. These include higher pacing thresholds and increased lead revision rate (1).
Upadhyay et al. have re-examined the mechanisms underpinning LBBB. In a group of 85 patients, half of whom were referred for a device implant and half of whom were referred for substrate mapping for ventricular tachycardia (VT) ablation, high-density intracardiac mapping of the left septum was performed (7). The majority of these patients had LBBB. In this study, left septal mapping was performed for the first time, to assess the presence and the level of block and the response to temporary HBP.
The cause of LBBB was found to be localized conduction block in 64% of cohort and no specific block but intraventricular conduction delay (IVCD) with intact Purkinje activation (IPA) in the remainder of the cohort. Those with conduction block either had block at the level of the His bundle at the left septum (72%) or proximally within the left bundle (28%). The majority of the patients with intra-hisian block responded to HBP (94%), compared to 64% of those with block in left bundle and none of the patients with IPA. Multiple electrocardiography (ECG) criteria were compared to look for any predictors of response to HBP, though mid QRS notching had a 100% negative predictive value (NPV), presence of conduction block offered the same NPV with a much higher positive predictive value (85% vs. 67%).
This study therefore confirmed key findings from previous studies that the majority of patients with broad LBBB (and low ejection fraction) have conduction disease at level of proximal His Purkinje system, associated with delayed transseptal conduction time (8) and that this can be successfully reversed with HBP. Based on left septal Purkinje activation, patients with LBBB morphology can be divided into two groups—those with delayed septal conduction (who have a broader QRS duration) and those with normal septal activation and delayed myocardial conduction (i.e., the group with IVCD or IPA) (8). However, the findings did dispute Narula’s concept of longitudinal dissociation with no evidence of differential conduction within the His bundle with the presence of intact fibres distal to the proximal conduction disease.
It is important to review the group of patients who were studied here. There were 2 distinct groups—those awaiting a CRT and those prior to substrate mapping and VT ablation. Overall the results suggest that the former group were more likely to have patients who had conduction block and the mapping group more likely to have patients with IPA or IVCD. These patients had lower ejection fraction, higher VT burden, greater use of amiodarone and higher numbers of ischemic cardiomyopathy. They probably represented a ‘sicker’ population potentially less likely to respond to HBP. Furthermore, there may have been an effect of amiodarone on the myocardium possibly leading to change in conduction and refractory properties. This may have also contributed to higher prevalence of IVCD.
When HBP was assessed, there was no fixation into the His bundle which might have led to a higher rate of LBBB reversal, though there was stimulation at the left side of the His bundle which would potentially enable conduction block in the proximal His and left bundle to be overcome more easily. It would have been interesting to know if the output needed to reverse conduction block at level of His was lower than at the level of left bundle.
In the current literature, where permanent HBP had been undertaken in such patients undergoing CRT, acute LBBB reversal rates have been higher than the 64% reported in this study, varying from 76% to 97% (5,6,9). A possible reason for this may have been the ability to deliver an active fixation lead into the His bundle. Additionally, stringent ECG criteria for LBBB were used in studies with high LBBB correction rates (6,9). There were also differences in how LBBB reversal was defined with two studies defining at least 20% reduction in intrinsic QRS duration needed and in this study an absolute value was used (130 ms from intrinsicoid R wave to end of QRS); however the average paced QRS duration in all these studies were lower than 130 ms (5,6,9). A recently published study also demonstrated 78% reversal rate of patients with right bundle branch block (RBBB) and low ejection fraction (10).
There is increasing interest in left bundle branch pacing (LBBP) (11,12) with the recent demonstration of pacing the left bundle beyond the site of block in the distal His bundle with associated reversal of ventricular dyssynchrony and improvement in left ventricle (LV) function. In patients in whom HBP requires high pacing output to correct LBBB, this new approach provides a very promising option to achieve left ventricular resynchronization at low and stable pacing output (Figures 1,2). Mechanistic understanding of LBBB as delineated in this study, clearly provides an opportunity to pace beyond the site of block in the His-Purkinje conduction system at the level of the proximal left bundle in the left septum. Deep septal LBBP by transvenous approach from the right ventricular septum would enable us to achieve high success rates of LBBB reversal in patients requiring CRT. Though intracardiac mapping may offer the best way of differentiating between the conduction block and IVCD, it is not practical during a device implant. Novel non-invasive methods of examining cardiac activation such as ECG imaging or refined ECG criteria may be helpful in patient selection of His-Purkinje conduction system pacing (13).
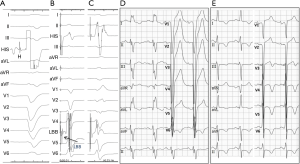
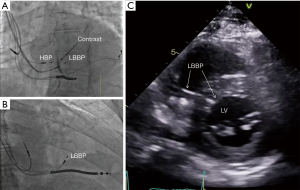
In conclusion, the future of His-Purkinje conduction system pacing will be determined by its evaluation in randomised controlled trials. It is also critical that tools for HBP are improved to allow for better outcomes including improvement in lead and sheath design and tailoring of pacing algorithms to allow for safe and appropriate His pacing (1,14). The authors of this study should be congratulated for the meticulous evaluation and their unique insights into the electrical basis of LBBB and assessment of principal determinants of success of HBP in LBBB reversal. Further mechanistic studies are required to assess the impact of distal His-Purkinje conduction system pacing including LBBP which can potentially lead to even greater success in LBBB reversal. With better understanding of the mechanism of LBBB and its correction by HBP, the future of conduction system pacing is brighter.
Acknowledgments
None.
Footnote
Conflicts of Interest: P Vijayaraman: speaker, consultant, research (Medtronic); consultant (Boston Scientific, Abbott, Merritt Medical); patent pending for his delivery tool. The other authors have no conflicts of interest to declare.
References
- Vijayaraman P, Chung MK, Dandamudi G, et al. His Bundle Pacing. J Am Coll Cardiol 2018;72:927-47. [Crossref] [PubMed]
- Sharma PS, Dandamudi G, Naperkowski A, et al. Permanent His-bundle pacing is feasible, safe, and superior to right ventricular pacing in routine clinical practice. Heart Rhythm 2015;12:305-12. [Crossref] [PubMed]
- Huang W, Su L, Wu S, et al. Benefits of Permanent His Bundle Pacing Combined With Atrioventricular Node Ablation in Atrial Fibrillation Patients With Heart Failure With Both Preserved and Reduced Left Ventricular Ejection Fraction. J Am Heart Assoc 2017;6. [Crossref] [PubMed]
- Narula OS. Longitudinal dissociation in the His bundle. Bundle branch block due to asynchronous conduction within the His bundle in man. Circulation 1977;56:996-1006. [Crossref] [PubMed]
- Lustgarten DL, Crespo EM, Arkhipova-Jenkins I, et al. His-bundle pacing versus biventricular pacing in cardiac resynchronization therapy patients: A crossover design comparison. Heart Rhythm 2015;12:1548-57. [Crossref] [PubMed]
- Sharma PS, Dandamudi G, Herweg B, et al. Permanent His-bundle pacing as an alternative to biventricular pacing for cardiac resynchronization therapy: A multicenter experience. Heart Rhythm 2018;15:413-20. [Crossref] [PubMed]
- Upadhyay GA, Cherian T, Shatz DY, et al. Intracardiac Delineation of Septal Conduction in Left Bundle-Branch Block Patterns. Circulation 2019;139:1876-88. [Crossref] [PubMed]
- Auricchio A, Fantoni C, Regoli F, et al. Characterization of left ventricular activation in patients with heart failure and left bundle-branch block. Circulation 2004;109:1133-9. [Crossref] [PubMed]
- Huang W, Su L, Wu S, et al. Long-term outcomes of His bundle pacing in patients with heart failure with left bundle branch block. Heart 2019;105:137-43. [Crossref] [PubMed]
- Sharma PS, Naperkowski A, Bauch TD, et al. Permanent His Bundle Pacing for Cardiac Resynchronization Therapy in Patients With Heart Failure and Right Bundle Branch Block. Circ Arrhythm Electrophysiol 2018;11:e006613. [Crossref] [PubMed]
- Huang W, Su L, Wu S, et al. A Novel Pacing Strategy With Low and Stable Output: Pacing the Left Bundle Branch Immediately Beyond the Conduction Block. Can J Cardiol 2017;33:1736.e1-1736.e3. [Crossref] [PubMed]
- Huang W, Su L, Wu S. Pacing Treatment of Atrial Fibrillation Patients with Heart Failure: His Bundle Pacing Combined with Atrioventricular Node Ablation. Card Electrophysiol Clin 2018;10:519-35. [Crossref] [PubMed]
- Arnold AD, Shun-Shin MJ, Keene D, et al. His Resynchronization Versus Biventricular Pacing in Patients With Heart Failure and Left Bundle Branch Block. J Am Coll Cardiol 2018;72:3112-22. [Crossref] [PubMed]
- Burri H, Keene D, Whinnett Z, et al. Device Programming for His Bundle Pacing. Circ Arrhythm Electrophysiol 2019;12:e006816. [Crossref] [PubMed]


