Transcatheter versus surgical aortic valve replacement in low and intermediate risk patients with severe aortic stenosis: systematic review and meta-analysis of randomized controlled trials and propensity score matching observational studies
Introduction
Aortic stenosis (AS) remains one of the major health concerns universally, with an increased prevalence due to the global aging population (1-3). When symptoms occur, the prognosis of severe AS is dismal (4) with 5-year survival rate of 15–50% (5). Surgical aortic valve replacement (SAVR) is a conventional treatment for management of severe AS for decades. Meanwhile, transcatheter aortic valve replacement (TAVR) becomes a popular alternative strategy in recent years. TAVR has been widely demonstrated to be comparable with SAVR (6,7), even better than TAVR to some extent among prohibitive and high risk population (8), and it is not an inferior management compared to SAVR in intermediate risk patients with severe AS (6,9-11). In addition, updated version 2017 AHA/ACC guidelines primarily recommend TAVR as an optimal method for intermediate risk patients due to recent studies (12,13). Although class of recommendation of TAVR (class IIa) is lower than SAVR (class I) for intermediate patients and recommending SAVR over TAVR in low risk patients in the current guidelines (12,14), half of Europe TAVR centers performed TAVR in intermediate risk patients and many of them did TAVR in low risk patients (15). A high quality randomized meta-analysis which was conducted in 2016 compared outcomes of TAVR with SAVR in low and intermediate risk patients (6). In 2017, another large-scale RCT study was carried out, two other meta-analysis (16,17) were conducted to compare outcomes of SAVR with TAVR as well, nevertheless, they failed to assess the quality of their evidence and were limited by smaller number of included studies. Therefore, this encouraged us to perform an updated systematic review and meta-analysis of RCT and PSM to compare performance of TAVR with SAVR in low and intermediate risk patients with severe AS.
Methods
Protocol
The registered systematic review protocol is available on PROSPERO (CRD 42018112626).
Literature resources
Two authors searched relevant literature independently based on the PICOS retrieval strategy in different databases including PubMed, Cochrane CENTRAL, EmBase, and Web of Science from 2002 to 30, September, 2018. The following key words were used alone or in combination: “transcatheter aortic valve replacement”, “surgical aortic valve replacement”, “low risk”, “intermediate risk”, “randomized controlled trials”, “propensity score matching”, “observational study”, or “aortic stenosis”. Some references in relevant studies were manually searched for additional articles which could not be identified through advance search.
Study selection
Inclusion criteria were: (I) study directly compared outcomes of TAVR with SAVR; (II) patients (≥18 years) whose mean Society of Thoracic Surgeons predicted risk of mortality (STS-PROM) ≤8% or mean European System for Cardiac Operative Risk Evaluation I (EuroSCORE I) ≤20% were selected in this review; (III) Articles reported at least one of the early and follow-up outcomes; (IV) RCT and PSM studies; (V) English studies. Exclusion criteria were: (I) patients with high and prohibitive risk; (II) non-randomized studies and other observational studies, non-PSM studies; (III) reviews, case reports; and (IV) non-English studies; the Valve Academic Research Consortium-2 (VARC-2) has been used in the selected RCT and PSM studies (18).
Data extraction process and analysis
Two authors extracted data independently from the included studies, via pre-standardized data collection forms, and any disagreements were resolved by consensus or through consulting a third author. The characteristics of all selected topics were extracted and categorized as following: number and baseline demographics of participants, year of publication, intervention details, duration of follow-up, mean STS-PROM, and EuroSCORE, early outcomes, and follow-up outcomes. A random-effect model was utilized to calculate risk ratio (RR) with corresponding 95% confidence intervals (95% CIs) for each dichotomous outcome. Data from RCT studies were analyzed separately from those of PSM studies.
Risk of bias and quality of evidence
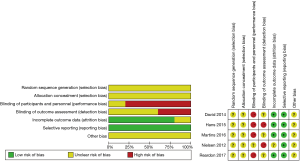
The Cochrane Collaboration’s tool for assessing risk of bias (19) was utilized to assess risk of bias of RCTs, moreover, the Newcastle-Ottawa Scale (NOS) (20) was used to assess the quality of PSM studies. Review Manager (version 5.3) and GRADE profiler 3.6 version were applied to perform meta-analysis and evaluate the overall quality of evidence respectively (21).
Results
Baseline demographic
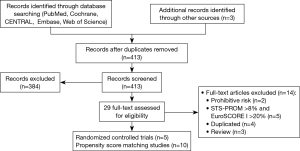
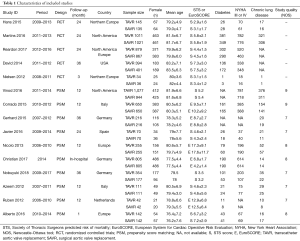
Our systematic literature search of electronic sources initially searched 1,427 records, and 3 additional records were identified from other sources. After de-duplication, a total of 413 titles and abstracts were assessed for eligibility. Then excluded 384 clearly irrelevant records, full-text articles of 29 records were obtained for further assessment. Eventually we included 5 RCTs (9,22-25) and 10 PSM studies (10,26-34). Flowchart of study selection is shown in Figure 1. Characteristics of included studies and assessments of PSM studies quality were illustrated in Table 1. Risk of bias in RCT studies is shown in Figure 2. Totally 12,057 patients were enrolled, out of that, 6,185 patients underwent TAVR procedure, and 5,872 patients for SAVR procedure. RCT studies enrolled 2,463 patients for TAVR versus 3,722 for SAVR, PSM studies included 2,460 patients for TAVR versus 3,412 patients for SAVR, respectively.
Full table
We defined all-cause mortality at 30 days, 1-, 2-, 3-year and disabling stroke at 30 days, 1-year as primary endpoints. Secondary outcomes were as followings: vascular complication, aortic valve (AV) re-intervention, aortic insufficiency (AI), major bleeding, permanent pacemaker implantation, myocardial infarction (MI), new-onset of atrial fibrillation (AF), acute kidney injury (AKI).
Primary endpoints
Mortality
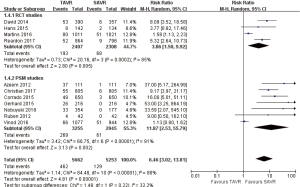
Pooled analysis of included studies illustrated that there was no significant statistical difference between all-cause mortality of TAVR and that of SAVR at 30 days, 1 year, 2, or 3 years.
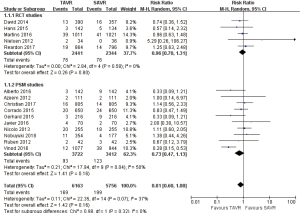
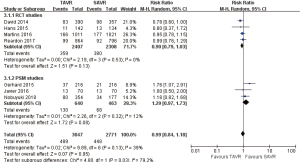
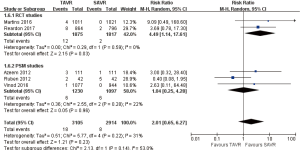
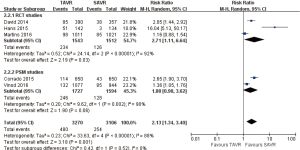
- 30 days’ comparison of 15 studies (RR 0.81, 95% CI: 0.60 to 1.08, Heterogeneity P=0.07, I2 =37%), this pooled result is in accordance with RCT studies and PSM studies separately (RCT: RR 0.96, 95% CI: 0.70 to 1.31, Heterogeneity P=0.58, I2 =0%; PSM: RR 0.73, 95% CI: 0.47 to 1.13, Heterogeneity P=0.04, I2 =50%, Figure 3).
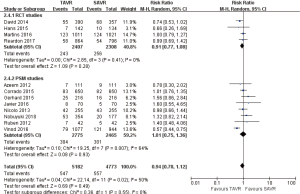
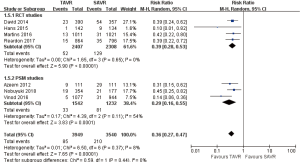
- 1-year comparison of 12 studies (RR 0.94, 95% CI: 0.78 to 1.12, Heterogeneity P=0.02, I2 =50%), this pooled result is in accordance with RCT studies and PSM studies separately (RCT: RR 0.91, 95% CI: 0.77 to 1.08, Heterogeneity P=0.41, I2 =0%; PSM: RR 1.01, 95% CI: 0.75 to 1.36, Heterogeneity P=0.007, I2 =64%, Figure 4).
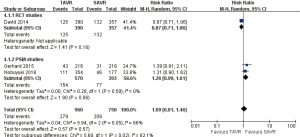
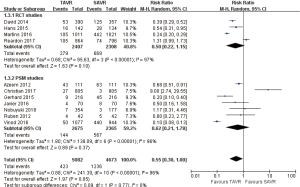
- 2-year comparison of 7 studies (RR 0.99, 95% CI: 0.84 to 1.18, Heterogeneity P=0.13, I2 =39%), this pooled result is in accordance with RCT studies and PSM studies separately (RCT: RR 0.90, 95% CI: 0.79 to 1.03, Heterogeneity P=0.53, I2 =0%; PSM: RR 1.29, 95% CI: 0.97 to 1.73, Heterogeneity P=0.32, I2 =12%, Figure 5).
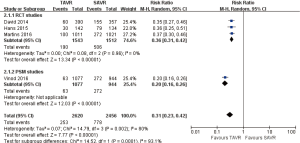
- 3-year comparison of 3 studies (1 RCT study) (RR 1.09, 95% CI: 0.81 to 1.46, Heterogeneity P=0.05, I2 =66%), this pooled result is in accordance with RCT studies and PSM studies separately (RCT: RR 0.87, 95% CI: 0.71 to 1.06; PSM: RR 1.26, 95% CI: 0.99 to 1.61, Heterogeneity P=0.59, I2 =0%, Figure 6).
Disabling stroke
Pooled analysis of the included studies revealed that, disabling stroke rate for TAVR was lower than that of SAVR at 30 days, however, there was no significant statistical difference between TAVR and SAVR at 1 year.
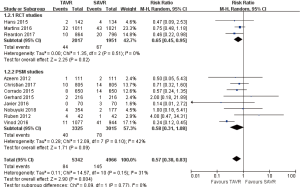
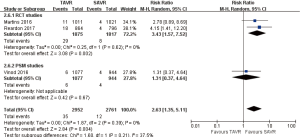
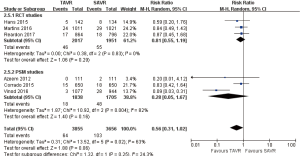
- 30 days comparison of 11 studies (RR 0.57, 95% CI: 0.38 to 0.83, Heterogeneity P=0.15, I2 =31%), this pooled result is in accordance with RCT studies (RCT: RR 0.65, 95% CI: 0.45 to 0.95, Heterogeneity P=0.51, I2 =0%), and PSM studies showed that, there was no significant difference between TAVR and SAVR (PSM: RR 0.58, 95% CI: 0.31 to 1.08, Heterogeneity P=0.10, I2 =42%, Figure 7).
- 1-year comparison of 6 studies (RR 0.70, 95% CI: 0.45 to 1.07, Heterogeneity P=0.01, I2 =65%), this pooled result is in accordance with RCT studies and PSM studies separately (RCT: RR 0.78, 95% CI: 0.55 to 1.11, Heterogeneity P=0.27, I2 =17%; PSM: RR 0.63, 95% CI: 0.27 to 1.46, Heterogeneity P=0.006, I2 =76%, Figure 8).
Secondary endpoints
AV re-intervention
Although pooled analysis of included studies demonstrated that, there was no statistical difference between aortic valve re-intervention of TAVR and SAVR at 30 days, yet the aortic valve re-intervention rate for TAVR was significantly higher than SAVR at 1-, 2-year during follow-up period.
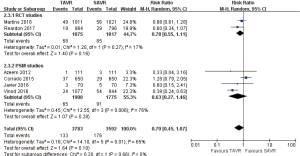
- 30 days comparison of 5 studies (RR 2.01, 95% CI: 0.65 to 6.27, Heterogeneity P=0.22, I2 =33%), this pooled result is in accordance with PSM studies (PSM: RR 1.04, 95% CI: 0.25 to 4.28, Heterogeneity P=0.28, I2 =22%), the rate of AV re-intervention for TAVR in RCT studies was significantly higher than that of SAVR (RCT: RR 4.49, 95% CI: 1.14 to 17.61, Heterogeneity P=0.59, I2 =0%, Figure 9).
- 1-year comparison of 3 studies (1 PSM study) (RR 2.63, 95% CI: 1.35 to 5.11, Heterogeneity P=0.39, I2 =0%), this pooled result is in accordance with RCT studies (RCT: RR 3.43, 95% CI: 1.57 to 7.52, Heterogeneity P=0.62, I2 =0%), and there was no statistically difference in PSM studies (PSM: RR 1.31, 95% CI: 0.37 to 4.64, Figure 10).
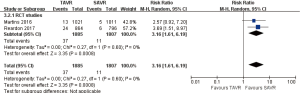
- 2-year comparison of 2 studies (2 RCT studies) (RR 3.16, 95% CI: 1.61 to 6.19, Heterogeneity P=0.60, I2 =0%, Figure 11).
days AI ≥ moderate (including paravalvular leakage PVL)
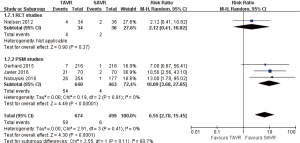
30 days comparison of 3 studies (1 RCT study) (RR 6.55, 95% CI: 2.78 to 15.45, Heterogeneity P=0.41, I2 =0%), this pooled result is in accordance with PSM studies (PSM: RR 10.09, 95% CI: 3.68 to 27.65, Heterogeneity P=0.91, I2 =0%), there was no statistically difference in RCT studies (RCT: RR 2.12, 95% CI: 0.41 to 10.82, Figure 12).
days vascular complications
Pooled analysis of 11 studies exposed that vascular complications rate for TAVR is significantly higher than that of SAVR at 30 days (RR 6.46, 95% CI: 3.02 to 13.81, Heterogeneity P<0.001, I2 =88%), this pooled result is in accordance with RCT studies and PSM studies separately (RCT: RR 3.86, 95% CI: 1.50 to 9.92, Heterogeneity P<0.001, I2 =85%; PSM: RR 11.87, 95% CI: 2.53 to 55.79, Heterogeneity P<0.001, I2 =91%, Figure 13).
days AKI
Pooled analysis of 7 studies disclosed that acute kidney injury rate for TAVR is significantly lower than SAVR at 30 days (RR 0.36, 95% CI: 0.27 to 0.47, Heterogeneity P=0.37, I2 =8%), this pooled result is in accordance with RCT studies and PSM studies separately (RCT: RR 0.39, 95% CI: 0.28 to 0.53, Heterogeneity P=0.65, I2 =0%; PSM: RR 0.29, 95% CI: 0.16 to 0.55, Heterogeneity P=0.11, I2 =54%, Figure 14).
days major bleeding
Pooled analysis of 11 studies revealed that there was no significant statistical difference between major bleeding of TAVR and SAVR at 30 days (RR 0.55, 95% CI: 0.30 to 1.00, Heterogeneity P<0.001, I2 =96%), this pooled result is in accordance with RCT studies and PSM studies separately (RCT: RR 0.5, 95% CI: 0.22 to 1.15, Heterogeneity P<0.001, I2 =97%; PSM: RR 0.62, 95% CI: 0.21 to 1.78, Heterogeneity P<0.001, I2 =96%, Figure 15).
1-year new onset AF
Pooled analysis of 4 studies (1 PSM study) illustrated that new onset of atrial fibrillation rate in TAVR is significantly lower than SAVR at 1-year (RR 0.31, 95% CI: 0.23 to 0.42, Heterogeneity P=0.002, I2 =80%), this pooled result is in accordance with RCT studies and PSM studies separately (RCT: RR 0.36, 95% CI: 0.31 to 0.42, Heterogeneity P=0.96, I2 =0%; Figure 16).
1-year permanent pacemaker implantation
Pooled analysis of 5 studies showed that permanent pacemaker implantation rate for TAVR is higher than SAVR at 1-year (RR 2.13, 95% CI: 1.34 to 3.40, Heterogeneity P<0.001, I2 =88%), this pooled result is in accordance with RCT studies and PSM studies separately (RCT: RR 2.71, 95% CI: 1.11 to 6.64, Heterogeneity P<0.001, I2 =92%; PSM: RR 1.88, 95% CI: 0.98 to 3.62, Heterogeneity P=0.002, I2 =90%, Figure 17).
1-year MI
Pooled analysis of 6 studies exposed that there was no significant statistical difference between myocardial infarction of TAVR and SAVR at 1 year (RR 0.56, 95% CI: 0.31 to 1.02, Heterogeneity P=0.02, I2 =63%), this pooled result is in accordance with RCT studies and PSM studies separately (RCT: RR 0.81, 95% CI: 0.55 to 1.19, Heterogeneity P=0.83, I2 =0%; PSM: RR 0.28, 95% CI: 0.05 to 1.67, Heterogeneity P=0.004, I2 =82%, Figure 18).
Low surgical risk patients’ results
Mortality
Furthermore, low risk patients were stratified for comparison between TAVR and SAVR, 3 of the included studies provided enough data to calculate pooled 30 days all-cause mortality. Pooled analysis showed that there was no significant statistical difference between TAVR and SAVR in aspect of 30 days mortality in low risk patients (RR 1.1, 95% CI: 0.64 to 1.90, Heterogeneity P=0.53, I2 =0%).
Disabling stroke
Three of the included studies provided enough data to calculate pooled 30 days disabling stroke. Pooled analysis showed there was no significant statistical difference between TAVR and SAVR in low risk patients for 30 days disabling stroke (RR 0.84, 95% CI: 0.43 to 1.64, Heterogeneity P=0.57, I2 =0%).
However, only one study reported 1, 2, 3 years follow-up results in low risk patients that SAVR is superior to TAVR for mortality and disabling stroke, these are needed to verify by developing further studies.
Heterogeneity analysis
Sensitivity analysis was conducted for the heterogeneity of outcome in which P<0.1 and I2>50%. In the aspects of 1-year disabling stroke, mortality, MI, 30 days vascular complication, the heterogeneity of these outcomes significantly decreased when we deleted one study (10) (P=0.81, I2 =0%, P=0.71, I2 =0%, P=0.36, I2 =0%, P=0.6, I2 =0%, respectively), this study is considered to be the source of the heterogeneity, but we can't delete it when considering the large sample size of this study. 30 days major bleeding, the heterogeneity didn’t change significantly when we deleted any one study, the result trend to be stable. 30 days vascular complication, the heterogeneity reduced remarkably (P=0.62, I2 =0%) when we deleted one RCT study (9), the surgical approach of 775 patients (75.9%) who underwent SAVR is trans-femoral, compared with other studies, this unconventional approach may be the reason for higher vascular complication which would result in high heterogeneity. And we also conducted subgroup analysis based on different study design.
Quality of evidence (GRADE)
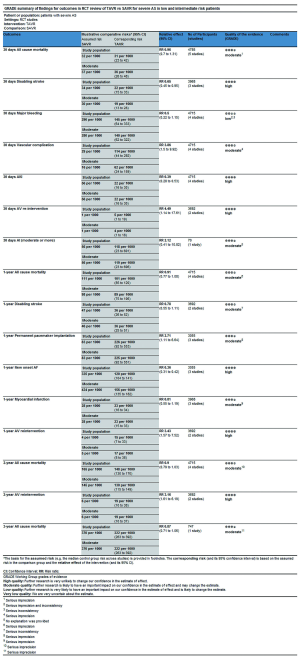
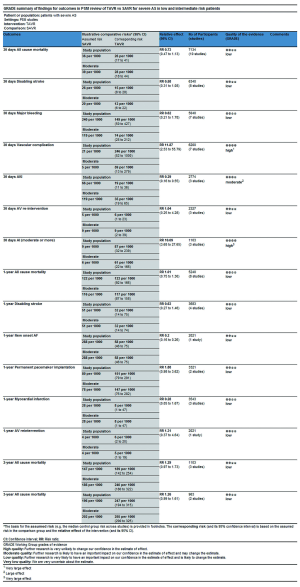
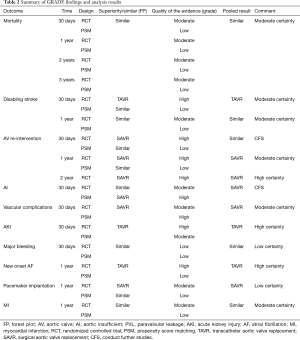
In this process, we defined primary endpoints as “critical outcome” and secondary endpoints as “important outcome”, and GRADE system was utilized to evaluate the quality of evidence from RCT studies (Figure S1) and PSM studies (Figure S2) separately according to GRADE handbook. Afterwards, we tried to combine GRADE findings with pooled results which were derived from forest plots to make comments for each outcome (shown in Table 2). The strategies were as follows: (I) if pooled results identified with both RCT results and PSM results, we regarded RCT GRADE finding as pooled results’ certainty; (II) if pooled results only identified with RCT while were not in accordance with PSM studies, we downgraded 1 level of RCT GRADE findings as pooled results’ certainty; (III) if pooled results only identified with PSM but not in accordance with RCT studies, we support to conduct further studies (CFS).
Full table
Discussion
First TAVR in human was performed by Alain Cribier in 2002 in France (35), during those years, TAVR became an important and popular alternative treatment for symptomatic severe AS patients, who were at prohibitive and high surgical risk (7,36-38). Updated version 2017 AHA/ACC guideline (12) has extended TAVR from high risk patients to intermediate risk patients compared to 2014 AHA/ACC guideline (13), however, 2017 ESC/EACTS guideline (14) conservatively recommends intermediate risk patients should be comprehensively evaluated by the heart team according to the individual patient characteristics when making decision between TAVR and SAVR. Therefore, we performed this updated systematic review to synthetically evaluate the performance of TAVR when compared with SAVR in low and intermediate risk population.
This review shows that mortality and disabling stroke during follow-up period are comparable between TAVR and SAVR (RCT: moderate certainty, PSM: low certainty), TAVR is superior to SAVR in aspects of AKI and new onset of AF (both RCT: high certainty, AKI in PSM: moderate certainty, AF in PSM: low certainty), meanwhile, SAVR is superior to TAVR in aspects of AV re-intervention, AI (including PVL), vascular complications, permanent pacemaker implantation according to RCT review. AV re-intervention and AI (moderate or more) are important indicators of evaluating durability of valve, at the same time, durability of TAVR valve is an important concern after TAVR, Matthew (39) has reported early failure cases of transcatheter aortic valves which including cusp rupture, valve thrombosis and accelerated calcification. The latest US Pivotal study (40) has reported 5-year freedom from severe structural valve deterioration of self-expand TAVR CoreValve is comparable with SAVR in high risk population, 5-year freedom from AV re-intervention is lower than SAVR, but both adverse events are uncommon in high risk patients. In spite of several available data reported excellent durability of TAVR valve, these are not enough to reduce concern about the durability because of insufficient follow-up time and restricted population, careful follow-up of all patients with TAVR valve and long-term valve deterioration assessment in low and intermediate risk patients, standardized definitions are warranted and will provide more information on both understanding and management of various forms of valve failure (41). From economic perspective, cost-utility of treatment may play a crucial role for patients who are from developing and low income countries. Although recent study (42) reported TAVR is cost-effective for the treatment of in severe AS patients at intermediate surgical risk, they remained moderate-to-high uncertainty surrounding the base-case incremental cost-effectiveness ratio. What’s more, many studies (29,43-45) have reported that cost associated with TAVR in operable population was significantly higher than SAVR regardless of intermediate or high risk patients, the difference was predominantly caused by higher transcatheter valve cost, SAVR may be an economically and clinically attractive treatment when taking the similar primary outcomes(mortality and disabling stroke) compared TAVR with SAVR and different costs into account for patients at low and intermediate risk who cannot afford to pay for costs. Patients, especially those who have absolute contraindication for SAVR or place a lower value on the risk of long-term valve failure, are more likely to obtain benefits from TAVR.
Strength and limitation
The strength of the review is that we included both RCT studies and PSM studies together. RCT is golden standard for evaluating intervention’s effectiveness and safety, however, PSM is an effective method for reducing confounding factors in observational study as well, this review analyzed a real-world data from PSM studies and avoided the possible selection bias of clinical trials. Other strengths of this review including a comprehensive search for relevant studies, independently extract data, assess eligibility, risk of bias, the quality of PSM studies and evidence separately based on the different study design and the credibility of subgroup analysis (RCT subgroup and PSM subgroup).
The limitation of this review included the followings: different generation transcatheter valve which may influence outcome of TAVR, such as AI, AV re-intervention. With the development of valve technology, more durable valve appears to reduce valve deterioration and AV re-intervention. Previous review (6) reported that transfemoral TAVR is superior to transapical TAVR versus SAVR in low and intermediate risk population, to a certain extent, TAVR approach is an important factor which can affect the outcome, we failed to grouping patients according to different intervention access due to the limited information in PSM studies, other limitation included publication bias, heterogeneity, heart team’s experience and skills.
Conclusions
TAVR is comparable to SAVR in terms of mortality and disabling stroke for severe AS patients at low and intermediate risk, but higher proportion of AV re-intervention was observed in TAVR. Those results should encourage caution when extending the indications of TAVR into low risk patients, especially for these young low risk patients, because of insufficient follow-up time to report the durability of TAVR valve.
Acknowledgments
Funding: This study was supported by International Cooperation and Exchange Project (NO. 81861128025).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Goldbarg SH, Elmariah S, Miller MA, et al. Insights into degenerative aortic valve disease. J Am Coll Cardiol 2007;50:1205-13. [Crossref] [PubMed]
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [Crossref] [PubMed]
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231-43. [Crossref] [PubMed]
- Carabello BA, Paulus WJ. Aortic stenosis. Lancet 2009;373:956-66. [Crossref] [PubMed]
- Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease(version 2012):the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2012;42:S1-44. [PubMed]
- Siemieniuk RA, Agoritsas T, Manja V, et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic stenosis at low and intermediate risk: systematic review and meta-analysis. BMJ 2016;354:i5130. [Crossref] [PubMed]
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement inhigh-risk patients. N Engl J Med 2011;364:2187-98. [Crossref] [PubMed]
- Fusari M, Bona V, Muratori M, et al. Transcatheter vs. surgical aortic valve replacement: a retrospective analysis assessing clinical effectiveness and safety. J Cardiovasc Med (Hagerstown) 2012;13:229-41. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609-20. [Crossref] [PubMed]
- Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 2016;387:2218-25. [Crossref] [PubMed]
- Foroutan F, Guyatt GH, O'Brien K, et al. Prognosis after surgical replacement with a bioprosthetic aortic valve in patients with severe symptomatic aortic stenosis: systematic review of observational studies. BMJ 2016;354:i5065. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease:A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70:252-89. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:e521-643. Erratum in Circulation 2014;130:e120. Dosage error in article text..
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739-91. [Crossref] [PubMed]
- Petronio AS, Capranzano P, Barbato E, et al. Current status of transcatheter valve therapy in Europe:results from an EAPCI survey. EuroIntervention 2016;12:890-5. [Crossref] [PubMed]
- Witberg G, Lador A, Yahav D, et al. Transcatheter versus surgical aortic valve replacement in patients at low surgical risk:A meta-analysis of randomized trials and propensity score matched observational studies. Catheter Cardiovasc Interv. 2018;92:408-16. [Crossref] [PubMed]
- Gargiulo G, Sannino A, Capodanno D, et al. Transcatheter Aortic Valve Implantation Versus Surgical Aortic Valve Replacement: A Systematic Review and Meta-analysis. Ann Intern Med 2016;165:334-44. [Crossref] [PubMed]
- Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Thorac Cardiovasc Surg 2013;145:6-23. [Crossref] [PubMed]
- Corbett MS, Higgins JP, Woolacott NF. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Res Synth Methods 2014;5:79-85. [Crossref] [PubMed]
- Deeks JJ, Dinnes J, D'Amico R, et al. Evaluating non-randomised intervention studies. Health Technol Assess 2003;7:iii-x, 1-173. [Crossref] [PubMed]
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. [Crossref] [PubMed]
- Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014;370:1790-8. [Crossref] [PubMed]
- Nielsen HH, Klaaborg KE, Nissen H, et al. A prospective, randomised trial of transapical transcatheter aortic valve implantation vs. surgical aortic valve replacement in operable elderly patients with aortic stenosis: the STACCATO trial. EuroIntervention 2012;8:383-9. [Crossref] [PubMed]
- Thyregod HG, Steinbruchel DA, Ihlemann N, et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Severe Aortic Valve Stenosis:1-Year Results From the All-Comers NOTION Randomized Clinical Trial. J Am Coll Cardiol 2015;65:2184-94. [Crossref] [PubMed]
- Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2017;376:1321-31. [Crossref] [PubMed]
- Frerker C, Bestehorn K, Schluter M, et al. In-hospital mortality in propensity-score matched low-risk patients undergoing routine isolated surgical or transfemoral transcatheter aortic valve replacement in 2014 in Germany. Clin Res Cardiol 2017;106:610-7. [Crossref] [PubMed]
- Furukawa N, Kuss O, Emmel E, et al. Minimally invasive versus transapical versus transfemoral aortic valve implantation: A one-to-one-to-one propensity score-matched analysis. J Thorac Cardiovasc Surg 2018;156:1825-34. [Crossref] [PubMed]
- Latib A, Maisano F, Bertoldi L, et al. Transcatheter vs surgical aortic valve replacement in intermediate-surgical-risk patients with aortic stenosis: a propensity score-matched case-control study. Am Heart J 2012;164:910-7. [Crossref] [PubMed]
- Osnabrugge RL, Head SJ, Genders TS, et al. Costs of transcatheter versus surgical aortic valve replacement in intermediate-risk patients. Ann Thorac Surg 2012;94:1954-60. [Crossref] [PubMed]
- Repossini A, Di Bacco L, Passaretti B, et al. Early hemodynamics and clinical outcomes of isolated aortic valve replacement with stentless or transcatheter valve in intermediate-risk patients. J Thorac Cardiovasc Surg 2017;153:549-58.e3. [Crossref] [PubMed]
- Tamburino C, Barbanti M, D'Errigo P, et al. 1-Year Outcomes After Transfemoral Transcatheter or Surgical Aortic Valve Replacement: Results From the Italian OBSERVANT Study. J Am Coll Cardiol 2015;66:804-12. [Crossref] [PubMed]
- Schymik G, Heimeshoff M, Bramlage P, et al. A comparison of transcatheter aortic valve implantation and surgical aortic valve replacement in 1,141 patients with severe symptomatic aortic stenosis and less than high risk. Catheter Cardiovasc Interv 2015;86:738-44. [Crossref] [PubMed]
- Castrodeza J, Amat-Santos IJ, Blanco M, et al. Propensity score matched comparison of transcatheter aortic valve implantation versus conventional surgery in intermediate and low risk aortic stenosis patients: A hint of real-world. Cardiol J 2016;23:541-51. [PubMed]
- Piazza N, Kalesan B, van Mieghem N, et al. A 3-center comparison of 1-year mortality outcomes between transcatheter aortic valve implantation and surgical aortic valve replacement on the basis of propensity score matching among intermediate-risk surgical patients. JACC Cardiovasc Interv 2013;6:443-51. [Crossref] [PubMed]
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002;106:3006-8. [Crossref] [PubMed]
- Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacementor surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1):a randomised controlled trial. Lancet 2015;385:2477-84. [Crossref] [PubMed]
- Popma JJ, Adams DH, Reardon MJ, et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol 2014;63:1972-81. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [Crossref] [PubMed]
- Summers MR, Cremer PC, Jaber WA. Three mechanisms of early failure of transcatheter aortic valves: Valve thrombosis, cusp rupture, and accelerated calcification. J Thorac Cardiovasc Surg 2017;153:e87-93. [Crossref] [PubMed]
- Gleason TG, Reardon MJ, Popma JJ, et al. Self-Expanding Transcatheter Aortic Valve Replacement or Surgical Valve Replacement in High-Risk Patients:5-Year Outcomes. J Am Coll Cardiol 2018;72:2687-96. [Crossref] [PubMed]
- Eltchaninoff H, Durand E, Barbanti M, et al. EuroIntervention 2018;14:B64-73. [Crossref]
- Tam DY, Hughes A, Fremes SE, et al. A cost-utility analysis of transcatheter versus surgical aortic valve replacement for the treatment of aortic stenosis in the population with intermediate surgical risk. J Thorac Cardiovasc Surg 2018;155:1978-88.e1. [Crossref] [PubMed]
- Armoiry X, Obadia JF, Pascal L, et al. Comparison of transcatheter versus surgical aortic valve implantation in high-risk patients: A nationwide study in France. J Thorac Cardiovasc Surg 2018;156:1017-25.e4. [Crossref] [PubMed]
- Ailawadi G, LaPar DJ, Speir AM, et al. Contemporary Costs Associated With Transcatheter Aortic Valve Replacement: A Propensity-Matched Cost Analysis. Ann Thorac Surg 2016;101:154-60, 160.
- Doble B, Blackhouse G, Goeree R, et al. Cost-effectiveness of the Edwards SAPIEN transcatheter heart valve compared with standard management and surgical aortic valve replacement in patients with severe symptomatic aortic stenosis: a Canadian perspective. J Thorac Cardiovasc Surg 2013;146:52-60.e3. [Crossref] [PubMed]