Use of ulinastatin was associated with reduced mortality in critically ill patients with sepsis
Introduction
Sepsis is defined as a systemic organ dysfunction caused by an over-activation of the inflammatory response to infections. Severe sepsis and septic shock are leading causes of morbidity and mortality in the intensive care unit (ICU) (1,2) and hence efforts are continuously being made to develop novel interventions for sepsis treatment (3-6). Nonetheless, sepsis remains a major challenge for clinicians despite these advances.
Sepsis results from an over-activation of the inflammatory response that is characterized by excessive secretion of pro-inflammatory cytokines such as interleukin (IL)-1, tumor necrosis factor-alpha, and IL-6. Therefore, pathways mediating the release and clearance of these cytokines could present potential targets for sepsis treatment. Ulinastatin is a serine protease inhibitor found in the blood and urine of humans. Animal studies have demonstrated anti-inflammatory effects of ulinastatin (7,8) and identified potential benefits for the management of multiple organ dysfunction induced by sepsis (9). Ulinastatin achieved amelioration of inflammatory damage by modulating the quantity and function of regulatory T cells (Tregs) via the Toll-like receptor 4 (TLR4)/nuclear factor-kappa B (NF-κB) signaling pathway (10). Nonetheless, clinical studies have yielded conflicting results regarding the effectiveness of ulinastatin in the treatment of sepsis. While some studies could demonstrate beneficial effects, others failed to corroborate such results (11), and these studies are often limited by the lack of an appropriate control of important confounders. We therefore set out to investigate the effectiveness of ulinastatin in the treatment of sepsis and hypothesized that this serine protease inhibitor reduces the mortality risk of critically ill septic patients.
Methods
Study population
This retrospective study was conducted between January 2014 and July 2017 in the Wuhu No. 2 People’s Hospital in China. All patients admitted to the ICU were screened for potential eligibility. Medical charts were independently reviewed by two senior intensivists with more than 10 years of clinical experience in the ICU. We involved two investigators to ensure that no eligible patient was overlooked. Any disagreement was resolved by discussing the respective cases with a third investigator. Patients who had sepsis on day 1 of ICU entry were included in the study. Sepsis was defined as organ dysfunction plus infection according to The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) (12). Organ dysfunction was defined as an increase in the Sequential Organ Failure Assessment (SOFA) score by two or more points. Infections were defined by the International Classification of Diseases (ICD-9) code (13). Information on organ dysfunction and infection was retrospectively extracted from the electronic healthcare records. Thus, we could apply the Sepsis-3 definition to patients that had been treated before the definition was issued. The following patients were excluded from the study: (I) pregnant women, (II) patients aged <18 years, (III) patients with contraindications for the use of ulinastatin such as allergy, (IV) terminally ill patients with a do-not-resuscitate (NDR) order, and (V) patients with sepsis that was treated in other hospitals for more than 3 days upon arrival in our hospital. The ethics committee of Wuhu No. 2 People’s Hospital approved the study protocol (approval number: 201804). Informed consent was waived due to the retrospective design of the study. All individual patient data were de-identified and stored in an encrypted computer. The study was conducted in accordance with the Declaration of Helsinki.
Clinical variables
Demographic data such as age and gender were included in the analysis. Severity of illness was assessed using the Acute Physiology and Chronic Health Evaluation (APACHE) II score and the SOFA score (14,15). We computed the APACHE II score by using variables obtained within 24 hours of ICU admission. If there were several measurements for a variable, the one associated with the maximum point score was employed. The types of patients included were emergency, surgical, and medical patients. Laboratory variables, such as procalcitonin (PCT), IL-6, C-reactive protein (CRP), platelet count, pro B-type natriuretic peptide (proBNP), and white blood cell (WBC) count, were recorded on day 0 and day 3 of ICU admission.
In terms of ulinastatin use, the dosage and times of initiation and discontinuation were recorded. There was no local practice guideline for the initiation of ulinastatin administration in our institution, and the decision was therefore left to the discretion of the attending physician. While there is no standard protocol for the dosage of ulinastatin, it was standardized in our institution to 200,000 U three times a day. We specified that vasopressors to assess were epinephrine, norepinephrine, and dopamine at a concentration of more than 5 µg/kg/min.
Outcomes
The primary outcome was mortality at 28 days after ICU admission. Secondary outcomes included duration of mechanical ventilation (MV) and ICU length of stay. If a patient returned to the ICU within 48 hours after release, the ICU length of stay was computed as the sum of both ICU stays. Similarly, if a patient had to be re-intubated within 48 hours of weaning from MV, the MV duration was computed as the sum of both sessions.
Statistical analysis
We assumed that the mortality rate was 0.5 in the control group and that the treatment could reduce the mortality rate to 0.34. The proportion of treated patients to control patients was 7:3. The type I error was 0.05, the type II error 0.2. The required sample size to reach statistical significance was 256. We included a total of 263 patients to account for potentially missing data.
Continuous variables were tested for their distribution (skewness and kurtosis). Data with normal distribution were expressed as mean and standard deviation and compared between survivors and non-survivors with the student’s t-test. Skewed data were expressed as median and interquartile range (IQR) and were compared between groups by the Mann-Whitney U test. Qualitive variables were expressed as numbers and percentages and were compared between groups with the Chi-square test or Fisher’s exact test, with p values reported for each comparison.
To adjust for confounding factors in this retrospective study, a multivariable logistic regression model was built with the binary mortality outcome as the response variable and vasopressor use, fluid balance, MV use, age, and SOFA score as potential confounders (16). Inflammatory biomarkers such as CRP, PCT, and WBC count have previously been associated with mortality outcome and were therefore considered as mediators of the effect of ulinastatin (17-19). Changes in these biomarkers on day 3 compared to day 1 were analyzed for both the treatment group and the control group. Model discrimination was determined by the area under the receiver operating characteristic (ROC) curve (20,21).
The R package Compare Baseline Characteristics Between Groups (CBCgrps) software was employed for all statistical analyses (version 3.4.3) (22). A two-tailed p value <0.05 was regarded as statistically significant (23).
Results
A total of 297 patients fulfilling the definition of Sepsis-3.0 were screened by reviewing their medical charts. Thirty-four of these patients met the exclusion criteria and were omitted from the study (2 pregnant women, 4 patients <18 years old, 13 patients with do-not-resuscitate orders, and 15 patients in a late stage of sepsis upon admission to our ICU, Figure 1). The remaining 263 patients included 162 survivors and 101 non-survivors, with an overall 28-day mortality of 38%.
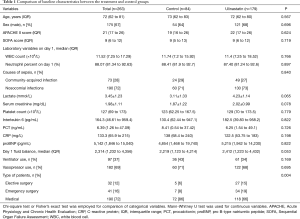
All variables except for the type of patient were comparable between the treatment and control groups (Table 1). In the treatment group, patients received ulinastatin for a median of 5 days (IQR: 3–11 days). We further compared baseline characteristics between survivors and non-survivors. As expected, non-survivors had higher APACHE II and SOFA scores than survivors (APACHE II: 24 for non-survivors, 19 for survivors; P<0.001; SOFA: 11 for non-survivors, 7 for survivors; P<0.001). Survivors were younger (median age of survivors: 70 years, median age of non-survivors: 77; P<0.001) and had a significantly higher WBC count on day 1 (survivors: 11.52×109/L, non-survivors: 10.95×109/L; P=0.034) than non-survivors. Other laboratory variables such as interleukin 6, PCT, CRP, proBNP, and platelet count did not significantly differ between both groups at baseline. Gender was not associated with mortality outcome (P=0.125). Non-survivors were more likely to use vasopressors (proportion for non-survivors: 0.88, survivors: 0.57; P<0.001), while the survivor group contained more surgical patients than the non-survivor group (survivors: 0.15, non-survivors: 0.08; P<0.001).
Full table
Table 2 shows the clinical outcomes of the treatment and control groups. There were 179 patients that received ulinastatin treatment during ICU stay and 84 control patients. Patients receiving ulinastatin showed a significantly lower mortality rate during the 28-day follow-up period (treatment group: 0.31, control group: 0.55; P<0.001). Nevertheless, patients in the treatment group experienced a longer duration of MV [treatment group: 3 days (IQR: 1–7 days), control group: 0 days (IQR: 0–3 days) in the control group; P<0.001], length of stay (LOS) in the ICU [treatment group: 5 days (IQR: 3–11 days), control group: 1 day (IQR: 0–6 days); P<0.001], and hospital stay [treatment group: 16 days (IQR: 7–27 days), control group: 10 days (IQR: 2–21 days); P<0.001] compared to the control group. The duration of vasopressor use did not significantly differ between both groups. Both CRP and PCT were significantly more reduced in the treatment group than in the control group.

Full table
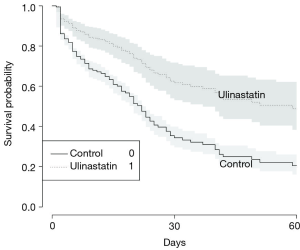
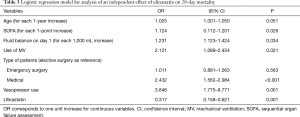
After adjustment for age, SOFA, vasopressor use, patient type, and fluid balance, the multivariable regression model revealed a significantly reduced risk of death associated with ulinastatin use (OR: 0.317, 95% CI: 0.158–0.621; P=0.001) (Table 3). Figure 1 shows the Kaplan-Meier curve for the treatment and control groups. The model discrimination was optimal as reflected by a C-index of 0.808.
Full table
Discussion
The present study demonstrates that ulinastatin treatment is associated with decreased 28-day mortality in critically ill septic patients. This association remained robust even after adjustment for the severity of illness as determined by the SOFA score. Nonetheless, our study also showed that ulinastatin was associated with prolonged ICU and hospital stays. We suggest that ulinastatin treatment may help ICU patients to survive the critical phase of sepsis. This group of patients typically requires a longer recovery time before discharge from the hospital.
Our findings are consistent with those of previous studies assessing the effect of ulinastatin on sepsis patients. Karnad et al. investigated ulinastatin treatment of 122 sepsis patients with one or more organ failures (24) and discovered that the 28-day all-cause mortality in the ulinastatin group was 7.3% (4 deaths) versus 20.3% (12 deaths) in the placebo group (P=0.045). The OR was 0.26 (95% CI: 0.07–0.95), which exceeds that reported in our study. Nevertheless, the results obtained in other studies do not agree with our observations. Uchida et al. found no association between ulinastatin treatment and 28-day mortality (OR: 1.22; 95% CI: 0.54–2.79) after adjustment for severity of illness and other confounding factors (11). These differences might reflect the higher age of the patients included in that particular study compared to those assessed in our study.
A proposed mechanism for the beneficial effect of ulinastatin is amelioration of the inflammatory response in sepsis patients. There is a large body of evidence from animal studies showing that ulinastatin treatment reduced inflammatory damage caused by sepsis (7,10,25,26). For example, Cao et al. reported that ulinastatin ameliorated inflammatory damage by modulating the quantity and function of Tregs via the TLR4/NF-κB signaling pathway (10); these biomarkers were not assessed in our clinical study. Nevertheless, we examined changes in inflammatory biomarkers such as CRP and PCT and observed that the levels of these biomarkers dropped to a greater extent in the treatment group than in the control group. Our findings therefore also support the previously observed anti-inflammatory properties of ulinastatin.
Zheng et al. performed a systematic review and meta-analysis of 16 studies (27) and found that treatment with ulinastatin in combination with Xuebijing (a Chinese patent medicine for the symptomatic treatment of sepsis, promoting blood circulation and preventing blood stasis) reduced the mortality rate [relative risk (RR) 0.54, 95% CI: 0.41–0.70; P<0.001], APACHE II score on day 7 [standardized mean difference (SMD) =−1.21, 95% CI: −1.62 to −0.80, P<0.01), duration of MV (SMD =−1.21, 95% CI: −1.62 to −0.80; P<0.01), and length of stay in the ICU (SMD =−1.21, 95% CI: −1.62 to −0.80; P<0.01). While the effect on mortality outcome was consistent with our study, we could not replicate the effects on MV duration and ICU length of stay. The concomitant use of Xuebijing (i.e., another agent with anti-inflammatory effects) in the study by Zheng et al. may lead to a synergistic effect of ulinastatin and Xuebijing in the treatment of critically ill patients with sepsis and explain the differences with our results (28,29). Combination of ulinastatin with other inflammation modulatory agents such as thymosin α1 that is known to restore immune function via enhancing cell-mediated immunity has proven promising in reducing mortality (30,31).
Several limitations of our study should be acknowledged. First, the retrospective design may result in selection bias. There might have been unmeasured confounders as patients receiving ulinastatin differed in many aspects from those in the control group. For example, we cannot exclude confounding by indication as the use of ulinastatin was at the discretion of the treating physician. The standard approach to adjust for such confounders is the use of a multivariable regression model, which we employed to incorporate such potential confounding factors such as age, SOFA, vasopressor use, and MV use. The results remained robust despite these adjustments. Nonetheless, certain unmeasured confounders cannot be addressed in observational studies and thus randomized controlled trials are mandatory to issue any recommendations for routine ulinastatin use. The ongoing ADJUST trial, a randomized controlled trial assessing the efficacy of ulinastatin compared to placebo in improving mortality outcome, will provide important evidence for such recommendations (32). A second limitation of our study was the lack of other anti-inflammatory agents such as Xuebijing and thymosin α1 that are not used in our hospital and we could therefore not determine any synergistic effects of these agents with ulinastatin.
Conclusions
In conclusion, we discovered that treatment with ulinastatin was associated with a decreased 28-day mortality in critically ill septic patients. Future randomized controlled trials are required before recommendations on ulinastatin use in critically ill patients can be issued.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The ethics committee of Wuhu No. 2 People’s Hospital approved the study protocol (approval number: 201804). Informed consent was waived due to the retrospective design of the study.
References
- Gaieski DF, Edwards JM, Kallan MJ, et al. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 2013;41:1167-74. [Crossref] [PubMed]
- Kaukonen KM, Bailey M, Suzuki S, et al. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA 2014;311:1308-16. [Crossref] [PubMed]
- Zhang Z, Smischney NJ, Zhang H, et al. AME evidence series 001-The Society for Translational Medicine: clinical practice guidelines for diagnosis and early identification of sepsis in the hospital. J Thorac Dis 2016;8:2654-65. [Crossref] [PubMed]
- Tao T, Zhao X, Lou J, et al. The top cited clinical research articles on sepsis: a bibliometric analysis. Crit Care 2012;16:R110. [Crossref] [PubMed]
- Angus DC, Barnato AE, Bell D, et al. A systematic review and meta-analysis of early goal-directed therapy for septic shock: the ARISE, ProCESS and ProMISe Investigators. Intensive Care Med 2015;41:1549-60. [Crossref] [PubMed]
- ARISE Investigators, ANZICS Clinical Trials Group, Peake SL, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014;371:1496-506. [Crossref] [PubMed]
- Cao C, Yin C, Shou S, et al. Ulinastatin Protects Against LPS-Induced Acute Lung Injury by Attenuating TLR4/NF-κB Pathway Activation and Reducing Inflammatory Mediators. Shock 2018;50:595-605. [Crossref] [PubMed]
- Luo Y, Che W, Zhao M. Ulinastatin post-treatment attenuates lipopolysaccharide-induced acute lung injury in rats and human alveolar epithelial cells. Int J Mol Med 2017;39:297-306. [Crossref] [PubMed]
- Atal SS, Atal S. Ulinastatin-a newer potential therapeutic option for multiple organ dysfunction syndrome. J Basic Clin Physiol Pharmacol 2016;27:91-9. [Crossref] [PubMed]
- Cao C, Yin C, Chai Y, et al. Ulinastatin mediates suppression of regulatory T cells through TLR4/NF-κB signaling pathway in murine sepsis. Int Immunopharmacol 2018;64:411-23. [Crossref] [PubMed]
- Uchida M, Abe T, Ono K, et al. Ulinastatin did not reduce mortality in elderly multiple organ failure patients: a retrospective observational study in a single center ICU. Acute Med Surg 2017;5:90-7. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Zhang Z, Hong Y. Development of a novel score for the prediction of hospital mortality in patients with severe sepsis: the use of electronic healthcare records with LASSO regression. Oncotarget 2017;8:49637-45. [PubMed]
- Knaus WA, Draper EA, Wagner DP, et al. APACHE II: A severity of disease classification system. Crit Care Med 1985;13:818-29. [Crossref] [PubMed]
- Minne L, Abu-Hanna A, de Jonge E. Evaluation of SOFA-based models for predicting mortality in the ICU: A systematic review. Crit Care 2008;12:R161. [Crossref] [PubMed]
- Zhang Z. Model building strategy for logistic regression: purposeful selection. Ann Transl Med 2016;4:111-1. [Crossref] [PubMed]
- Shukeri WFWM, Ralib AM, Abdulah NZ, et al. Sepsis mortality score for the prediction of mortality in septic patients. J Crit Care 2018;43:163-8. [Crossref] [PubMed]
- Zhang Z, Ni H. C-reactive protein as a predictor of mortality in critically ill patients: a meta-analysis and systematic review. Anaesth Intensive Care 2011;39:854-61. [Crossref] [PubMed]
- MacKay GJ, Molloy RG, O'Dwyer PJ. C-reactive protein as a predictor of postoperative infective complications following elective colorectal resection. Colorectal Dis 2011;13:583-7. [Crossref] [PubMed]
- Tolles J, Meurer WJ. Logistic Regression: Relating Patient Characteristics to Outcomes. JAMA 2016;316:533-4. [Crossref] [PubMed]
- Harrell FE. Regression Modeling Strategies. New York, NY: Springer New York, 2001.
- Zhang Z, Gayle AA, Wang J, et al. Comparing baseline characteristics between groups: an introduction to the CBCgrps package. Ann Transl Med 2017;5:484-4. [Crossref] [PubMed]
- Zhang Z. Data management by using R: big data clinical research series. Ann Transl Med 2015;3:303. [PubMed]
- Karnad DR, Bhadade R, Verma PK, et al. Intravenous administration of ulinastatin (human urinary trypsin inhibitor) in severe sepsis: A multicenter randomized controlled study. Intensive Care Med 2014;40:830-8. [Crossref] [PubMed]
- Liu S, Wei F, Luo L, et al. Ulinastatin attenuates hyper-permeability of vascular endothelialium cells induced by serum from patients with sepsis. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2017;33:1600-4. [PubMed]
- Li ST, Dai Q, Zhang SX, et al. Ulinastatin attenuates LPS-induced inflammation in mouse macrophage RAW264.7 cells by inhibiting the JNK/NF-κB signaling pathway and activating the PI3K/Akt/Nrf2 pathway. Acta Pharmacol Sin 2018;8:41988.
- Zheng J, Xiang X, Xiao B, et al. Xuebijing combined with ulinastation benefits patients with sepsis: A meta-analysis. Am J Emerg Med 2018;36:480-7. [Crossref] [PubMed]
- Shi H, Hong Y, Qian J, et al. Xuebijing in the treatment of patients with sepsis. Am J Emerg Med 2017;35:285-91. [Crossref] [PubMed]
- Gong P, Lu Z, Xing J, et al. Traditional Chinese medicine Xuebijing treatment is associated with decreased mortality risk of patients with moderate paraquat poisoning. PLoS One 2015;10:e0123504. [Crossref] [PubMed]
- Liu F, Wang HM, Wang T, et al. The efficacy of thymosin α1 as immunomodulatory treatment for sepsis: a systematic review of randomized controlled trials. BMC Infect Dis 2016;16:488. [Crossref] [PubMed]
- Liu D, Yu Z, Yin J, et al. Effect of ulinastatin combined with thymosin alpha1 on sepsis: A systematic review and meta-analysis of Chinese and Indian patients. J Crit Care 2017;39:259-66. [Crossref] [PubMed]
- Jiang W, Yu X, Sun T, et al. ADJunctive Ulinastatin in Sepsis Treatment in China (ADJUST study): study protocol for a randomized controlled trial. Trials 2018;19:133. [Crossref] [PubMed]