
Radical radiotherapy for locally advanced non-small cell lung cancer—what’s up with arm positioning?
Introduction
The treatment of stage III locally advanced non-small cell lung cancer (NSCLC) often includes concurrent chemotherapy and radiotherapy (RT) (1). Intensity-modulated radiation therapy (IMRT) or volumetric modulated arc therapy (VMAT) techniques may be used in this scenario to improve target dose conformity and organ-at-risk (OAR) sparing (2-4). Secondary analysis of a large randomized trial, supports routine use of IMRT for locally advanced NSCLC based on lower rates of severe pneumonitis, better quality of life, and lower cardiac doses compared to 3-dimensional conformal techniques (5).
Regardless of delivery method, RT is typically delivered in the arms-up (AU) position, so as to avoid restricting beam angles and unnecessary treatment through the arms. However, as lung cancer is often a disease of the elderly and comorbid, tolerability of the AU position for a prolonged course may be challenging. Furthermore, patient discomfort may lead to difficulties in position reproducibility and stability, requiring repeat simulation and planning in some cases. Where resources are limited, this process may lead to delays in treatment.
Comparable plan quality has been demonstrated in both the AU and arms-down (AD) position with stereotactic ablative radiotherapy (SABR) of a small solitary target in the context of early stage lung cancers using VMAT (6). The impact of changing arm position during RT for multiple and larger targets in more advanced lung cancers is not currently well described in the literature. Therefore, the objectives of this study are to: (I) determine the dosimetric impact of changing arm position during treatment, without re-planning, in stage III NSCLC patients; and (II) to compare the quality of RT plans in the AU position with re-optimized plans in a simulated AD position.
Methods
Cohort characteristics
In this Western University health research ethics board approved study (110806), ten patients with AJCC 8th edition stage III NSCLC and treated with RT between May 2016 and May 2017, were identified from our institutional database. Clinical stages were as follows: 1 patient with T4N0; 3 patients with T1N2; 3 patients with T2N2; and 3 patients with T3N2. Primary disease locations were in the right upper lobe (5 patients), left upper lobe (4 patients), and right lower lobe (1 patient); most cases (9/10) also included nodal volumes. Patients with supraclavicular lymph nodes included in the treatment volume were excluded. Clinically delivered treatment was 60 Gy in 30 fractions in the AU position using VMAT, per our institutional standard.
CT simulation
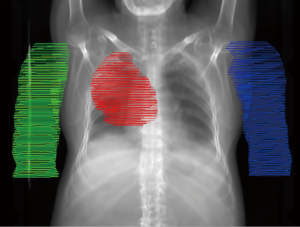
Patients were immobilized in the supine AU position using a Vac-lok. Fast helical and 4D CT-SIM scans were acquired and transferred to the Pinnacle treatment planning system (software version 9.10, Philips Healthcare, Fitchburg, WI, USA). To account for respiratory motion, the internal gross target volume (IGTV) was defined as the union of GTV contours on maximum inspiration and maximum expiration images. The IGTV was expanded by 8 mm, respecting anatomical boundaries, to create an internal target volume (ITV) and this was again expanded by 5 mm to create the planning target volume (PTV). The heart was also defined as the combination of contours at maximum inspiration and maximum expiration, and all other organs at risk were contoured on the untagged average dataset. An evaluation structure was created for total lung that excluded the IGTV; herein reported as ‘lung’.
Treatment planning
To retrospectively simulate AD treatment, a diagnostic PET/CT (acquired in the AD position) was rigidly registered, based on bony anatomy, to the untagged average CT used for planning. The arms were delineated and the density was manually overridden to 1 g/cm3 within each contour, as required. An example case is depicted in the supplementary appendix online (Figure S1). The clinically delivered VMAT plan was recalculated on new anatomy that included the density override for contralateral arm only, ipsilateral arm only, or both arms. Plans were also re-optimized for the AD position, simulated with density override on both arm contours, using VMAT with the same field size and arc range as the clinically delivered plan. During the optimization process, arms were treated as an OAR with dose constraints. The following dose-volume histogram (DVH) parameters were compared for each scenario: PTV D95, D99, and Dmax; Spinal cord Dmax; Esophagus Dmax and Dmean; Heart Dmean, V5, V25; Lung Dmean V5, V10, V20; Arm Dmax, Dmean, D1cc, D2cc, D5cc, D10cc. The treatment planning dose objectives for clinical and re-optimized plans are reported in Table 1.
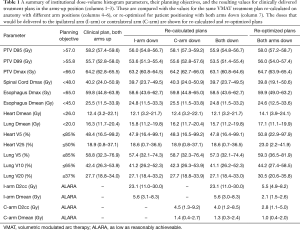
Full table
Statistical analysis
DVH parameters for clinical plans optimized with AU and re-optimized plans with AD were compared using two-sided paired t-tests or Wilcoxon matched-pairs signed rank tests, as appropriate, with a threshold of P<0.001 for statistical significance after adjusting for multiplicity testing. Monitor units (MU) required to deliver each plan were compared using a two-sided Mann-Whitney test with a threshold of P<0.05. Statistical analysis was performed using GraphPad Prism 8.
Results
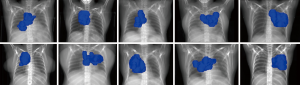
The mean (range) IGTV and PTV volumes were 170.5 (12.8–383.1) and 696.7 (483.8–1,150.8) cm3, respectively, with a mean 6.7% (3.4–12.7%) of lung volume overlapped by PTV. The location and relative laterality of each PTV is illustrated in Figure 1. The mean simulated arm volume was 1,384 [1,113–1,793] cm3. Partial arcs were used for the majority of patients in this study (7/10) to minimize contralateral lung dose. The median arc range was 225° [210–360°] and all plans used 6 MV photons.
Changing arm position without re-planning
DVH parameters for each arm scenario are reported in Table 1. Moving from AU to AD for the entire treatment course, without re-planning, reduced the PTV D95 by 3.7% (1.7–6.2%). In all cases, this caused PTV D95 to be less than 57 Gy [mean: 55.9 Gy (54.8–56.7 Gy)] and PTV D99 was reduced by 3.6% (1.6–6.4%) to 53.5 Gy (51.4–55.4 Gy), both of which did not meet our institutional objectives.
All other DVH parameters, including to OAR, on average were within 2% of the clinically delivered plan. The mean D2cc and Dmean to the ipsilateral arm were 23.1 (11.0–30.0) and 5.6 (3.0–8.3) Gy, respectively. Lateral tumor location was associated with higher doses to small volumes of the ipsilateral arm compared with more centrally located tumors. The contralateral arm received mean D2cc and Dmean of 4.0 (1.2–8.5) and 1.3 (0.3–2.4) Gy. Additional doses to small volumes of the arms can be found in Table S1. The dosimetric consequences of moving only the ipsilateral arm down were similar to both arms down, whereas contralateral arm only had less than 1% effect on PTV D95 and all other DVH parameters.
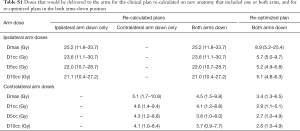
Full table
Planning in the arms down position
When plans were re-optimized to account for the both AD position, PTV D95 coverage was recovered with acceptable doses to all OAR. The differences between the clinically delivered plan in the AU position and the re-optimized plan in the AD position were minimal and are illustrated in Figure 2. There was a statistically significant difference in heart V25 and mean heart dose even after adjusting for multiplicity testing (P<0.001). Lateral angle restriction to reduce dose to the arms in the re-optimized plans caused more anterior-posterior dose deposition, resulting in increased heart dose compared with the clinical AU plan; however, the magnitude of the difference was small at 4.1% (1.4–8.4%) for V25 and 1.7 (0.7–3.8 Gy) for mean heart dose and the plans still met our institutional dose objectives (Table 1). Heart dose could be further reduced at a cost of increased dose to the arms.
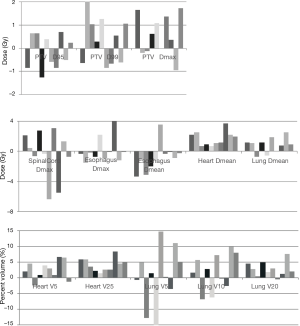
The arm doses were decreased after re-optimization compared with the re-calculated plans. The mean D2cc and Dmean for the ipsilateral arm were 5.5 (4.9–8.2) and 2.1 (1.5–2.6) Gy, respectively (Table 1). The contralateral arm D2cc and Dmean were 2.8 (1.1–5.0) and 1.0 (0.4–2.0) Gy. The mean total MU required to deliver the re-planned cases was 515 [366–797] and was not significantly different from clinical plans using 546 MU [288–794].
Discussion
These simulation results demonstrate the AD position did not cause a dosimetric disadvantage in treatment planning for stage III NSCLC patients. Achieving DVH objectives with arm avoidance was successful and did not require extensive field modulation. Overall, plans optimized in the AD position were of comparable quality to clinical plans created in the AU position. Of note, there was a statistically significant, but likely clinically insignificant, increase in heart dose; this may be mitigated by increasing the arc range but was not investigated in this study. Lung V20 was not statistically different between AU and AD plans, but should be considered on an individual basis given that a large meta-analysis demonstrated 3% increased risk of radiation pneumonitis per 1% increase in lung V20 (7).
The results also suggest, if necessary, a patient simulated and planned in the AU position could move only the contralateral arm down at any time during treatment without substantial compromise to the intended dosimetry and the delivered dose would be within 1% of the clinical plan. This is especially relevant if partial arcs were used and there is no entrance dose through the contralateral arm. It may also be reasonable to move and immobilize the ipsilateral arm alone, or both arms, in the AD position without re-planning depending on how many fractions remain. In the last week of treatment, changing to the AD position would result in less than 1% decrease in PTV D95 and this is unlikely to be substantially affected by potential variation in arm position over 30 fractions, as Shultz et al. previously demonstrated SABR plans were insensitive to 2.5 cm arm shifts (6). This is predicated on the assumption that image-guided radiotherapy (IGRT) allows for an adequate match to the target and OAR of concern after moving arm position. Practically, this may be difficult for tumors located in the lung apex, where changes in arm position will have a greater impact on the position of local anatomy.
Treating in the AD position inevitably results in unavoidable doses to the arms; however, acute or late toxicity to the arms would be expected to be rare at the dose levels reported in this study. For the worst case included, where the PTV extended laterally into the chest wall, the maximum dose to 2 cc of the ipsilateral arm was 30 Gy over 30 fractions if the plan was not re-optimized to account for the arm. For context, the equivalent dose in 2 Gy per fraction (EQD2) is 24 Gy (α/β=3 for normal tissue) and is less than the EQD2 of a palliative 20 Gy in 5 fractions treatment (EQD2=28 Gy); moreover, this estimate assumes arm position was changed for the entire treatment duration which is unlikely to occur without re-CT and plan re-optimization. In RTOG 0630, an extremity sarcoma trial with an RT prescription of 50 Gy in 25 fractions, the reported rate of grade ≥2 toxicity at 2 years was 10.5%. In this study with significantly higher RT doses to extremities than in the present study, constraints allowing up to 50% of a longitudinal strip of skin and subcutaneous tissue receiving 20 Gy (EQD2=15 Gy) were mandated (8). Regarding late effects, the risk of secondary malignancy attributable to arms within the treatment field is also likely to be exceedingly low (9,10). Still, clinical judgment is required to assess the tradeoff between arm dose and potential treatment delay for re-planning.
There are limitations to consider when interpreting these results. First, the sample size in this proof-of-concept study was small; however, efforts were made to include diversity in tumor locations. Figure 1 demonstrates a variety of lateral and medial PTV positions, but upper lobe disease comprises the majority of this cohort. In addition, the dose estimates may be limited by imperfect simulation of the AD position. Digital addition of the arms may not be completely spatially accurate, especially near the shoulders, and tissue heterogeneity within the arms was not considered during dose calculation. Moreover, movement of the arm position may lead to additional changes in thoracic anatomy that were not considered in this analysis.
Conclusions
This simulated planning study suggests that it is feasible to plan radiotherapy for locally advanced lung cancer patients in the arms down position using VMAT with only a modest dosimetric impact, when necessary. If an AU position is used for planning, but cannot be maintained on treatment, it may be reasonable to change arm position without re-planning near the end of the course, provided the IGRT match remains consistent.
Acknowledgments
Dr. Louie is supported by funding from the Ontario Association of Radiation Oncologists Clinician Scientist program. Dr. Palma is supported by a Clinician-Scientist Grant from the Ontario Institute for Cancer Research.
Footnote
Conflicts of Interest: Dr. Louie has received honoraria from Varian Medical Systems Inc. The other authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by Western University health research ethics board (No. 110806).
References
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer. NCCN Clin Pract Guidel Oncol [Internet]. 2018;(version 3.2018). Available online: https://www.nccn.org/professionals/physician_gls/default.aspx
- Grills IS, Yan D, Martinez AA, et al. Potential for reduced toxicity and dose escalation in the treatment of inoperable non-small-cell lung cancer: a comparison of intensity-modulated radiation therapy (IMRT), 3D conformal radiation, and elective nodal irradiation. Int J Radiat Oncol Biol Phys 2003;57:875-90. [Crossref] [PubMed]
- Christian JA, Bedford JL, Webb S, et al. Comparison of inverse-planned three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2007;67:735-41. [Crossref] [PubMed]
- Wijsman R, Dankers F, Troost EG, et al. Comparison of toxicity and outcome in advanced stage non-small cell lung cancer patients treated with intensity-modulated (chemo-)radiotherapy using IMRT or VMAT. Radiother Oncol 2017;122:295-9. [Crossref] [PubMed]
- Chun SG, Hu C, Choy H, et al. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non-Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J Clin Oncol 2017;35:56-62. [Crossref] [PubMed]
- Shultz DB, Jang SS, Hanlon AL, et al. The effect of arm position on the dosimetry of thoracic stereotactic ablative radiation therapy using volumetric modulated arc therapy. Pract Radiat Oncol 2014;4:192-7. [Crossref] [PubMed]
- Palma DA, Senan S, Tsujino K, et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 2013;85:444-50. [Crossref] [PubMed]
- Wang D, Zhang Q, Eisenberg BL, et al. Significant Reduction of Late Toxicities in Patients With Extremity Sarcoma Treated With Image-Guided Radiation Therapy to a Reduced Target Volume: Results of Radiation Therapy Oncology Group RTOG-0630 Trial. J Clin Oncol 2015;33:2231-8. [Crossref] [PubMed]
- Berrington de Gonzalez A, Curtis RE, Kry SF, et al. Proportion of second cancers attributable to radiotherapy treatment in adults: a cohort study in the US SEER cancer registries. Lancet Oncol 2011;12:353-60. [Crossref] [PubMed]
- Kirova YM, Gambotti L, De Rycke Y, et al. Risk of second malignancies after adjuvant radiotherapy for breast cancer: a large-scale, single-institution review. Int J Radiat Oncol Biol Phys 2007;68:359-63. [Crossref] [PubMed]


