Effects of preoperative sarcopenia on postoperative complications of minimally invasive oesophagectomy for oesophageal squamous cell carcinoma
Introduction
Oesophagectomy is one of the most traumatic surgical procedures in thoracic surgeries. Therefore, it is important to accurately predict postoperative complications and implement appropriate precautions. Although improvements in surgical techniques and the application of rapid rehabilitation (1) have reduced the incidence of postoperative complications of oesophageal cancer (2), the incidence remains high (≤60%) (3). Therefore, preoperative assessment of surgical risks and adjustment of perioperative nursing measures are important to reduce the incidence and mortality of complications.
Sarcopenia refers to a decrease in skeletal muscle mass, accompanied by a decrease in skeletal muscle strength or physical activity (4). Sarcopenia is a prognostic factor for postoperative complications and prognosis in patients with diverse malignant tumours and is thus a useful indicator in preoperative analysis and evaluation (5). Moreover, sarcopenia serves as an important predictor of postoperative complications of cancers of the liver, colon, pancreas and bladder (6-9).
We conducted a single centre, retrospective study to determine the association between preoperative sarcopenia and postoperative complications in patients undergoing minimally invasive oesophagectomy (MIE). We also investigated the relationships between postoperative complications and patients’ clinical characteristics such as age, body mass index (BMI) and lung function to identify potentially correctable abnormal clinical parameters that could help to reduce postoperative complications and improve patients’ quality of life.
Methods
Patients
We retrospectively analysed the clinical data for patients with oesophageal squamous cell carcinoma who underwent thoracoscopy combined with laparoscopic radical oesophagectomy at the Department of Thoracic Surgery, Fujian Medical University Union Hospital from December 2015 to October 2018. These patients were diagnosed with oesophageal squamous cell carcinoma using an electronic gastroscope before surgery. Each patient underwent chest and abdominal computed tomography (CT), oesophageal angiography, ultrasonography of the abdomen, neck and clavicle, and positron emission tomography-CT was performed if necessary.
The inclusion criteria for this study were as follows: lesion in the thoracic segment; preoperative staging cT1-3N0-1M0; undetectable distant metastasis; preoperative evaluation of the feasibility of minimally invasive surgery; except for other malignancies; no preoperative chemotherapy or radiotherapy; no concurrent severe diseases of the heart, liver, lung, kidney or other surgical contraindications; patients judged to tolerate surgical treatment. The study was approved by the Ethics Committee of Fujian Medical University and all participants gave informed consent before taking part.
Surgical procedures and perioperative nursing
The standard surgical procedure for thoracic oesophageal squamous cell carcinoma was thoracoscopic combined with laparoscopic radical resection, and the surgical procedures were carried out as follows. The patient was placed in the left semi-prone position, the thoracic oesophagus was completely freed, the lymph nodes were dissected along the bilateral recurrent laryngeal nerves, and the lymph nodes in the paraesophageal regions were dissected. The stomach, abdominal oesophagus, and cervical oesophagus were then freed and the perigastric lymph nodes were dissected. A 5-cm incision was made in the centre of the abdomen, the stomach was removed and shaped into a gastric tube, and a mechanical anastomosis was created with the cervical oesophagus. If three-field lymph node dissection was performed, the cervical lymph nodes were also dissected.
For perioperative nursing of patients with oesophageal cancer, we adopted improved perioperative management techniques. Preoperative preparation included nutritional support education, prohibition of smoking before surgery, enhanced oral care, and provision of effective information and education about discharging sputum from the lungs and preoperative retention of a peripherally inserted central catheter. Intraoperative attention was paid to protecting of the bilateral recurrent laryngeal nerve, appropriate use of energy instruments, and ensuring strict haemostasis and intraoperative airway management. Conventionally, indwelling jejunostomy tubes were used during surgery to administer postoperative enhanced enteral nutritional support. Routine postoperative management included use of drugs to improve the microcirculation, strengthen bedside endoscopic sputum aspiration and other measures to manage the respiratory tract, early implementation of enteral nutritional support, routine gastrointestinal decompression to reduce reflux aspiration and effective analgesia.
Diagnosis of sarcopenia
Preoperative CT was routinely performed to determine the size and location of the tumour and to evaluate the skeletal muscle mass at the level of the third lumbar spine (L3). If the patient underwent multiple CT examinations, the most recent preoperative data were used. Skeletal muscle coverage was defined using a −29 to 150 HU thresholds. The L3 region contains the psoas muscle, paraspinal muscles (erector spinae and quadratus lumborum) and abdominal wall muscles (transversus abdominis, external and internal obliques and rectus abdominis). The L3 level of skeletal muscle mass is proportional to the whole-body muscle mass. The skeletal muscle index (SMI) (cm2/m2) was defined as the normalized skeletal muscle area [height (m2)]. The present study was performed in accordance with Prado et al. (10) who defined the threshold of SMI for sarcopenia in men as 52.4 cm2/m2 and in women as 38.5 cm2/m2.
Postoperative complications
The diagnosis of postoperative complications conformed to the international consensus published by the Esophagectomy Complications Consensus Group (11). According to the consensus, postoperative complications can be classified as involving the lungs, heart, stomach, infected tissues, nerves and thromboembolism. Severity was graded according to the Clavien-Dindo classification (12). Patients with Grade II or higher adverse events occurring within 30 days after surgery or during hospitalization were considered to have complications.
Statistical analysis
Data were analysed using SPSS 22.0 software. Measured data were expressed as mean ± standard deviation (SD) and differences between groups were analysed by t-tests. Counted data were expressed as number or percent, and differences were analysed using χ2 or Fisher’s exact tests. Variables with P<0.10 in univariate analysis were further analysed by multivariate logistic regression. P<0.05 was considered statistically significant.
Results
General clinical data and grouping
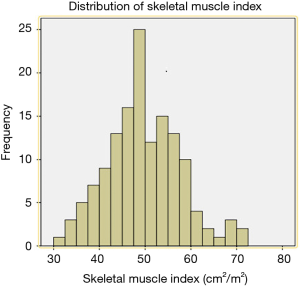
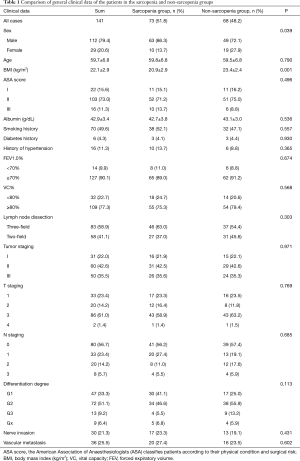
A total of 143 patients were included in the study. Two patients were excluded because their postoperative pathology results indicated adenocarcinoma and high-grade intraepithelial neoplasia, respectively. A total of 141 patients were therefore finally enrolled in our study. SMI values were as follows (Figure 1): mean, 49.5 cm2/m2; median, 49.3 cm2/m2; standard deviation (SD), 9.0 cm2/m2. The average SMI values for men and women were 51.4±8.7 and 42.4±6.6 cm2/m2, respectively. According to the above SMI threshold value, patients were divided into the sarcopenia (n=73) and non-sarcopenia (n=68) groups. The clinical characteristics of the two groups are summarized in Table 1. The sarcopenia group included a higher proportion of men (P=0.039) and had a lower mean BMI (P=0.001) than the non-sarcopenia group. There were no significant differences between variables such as vital capacity (VC), forced expiratory volume in the first second as a percent of forced vital capacity (FEV1.0%), American Society of Anaesthesiologist (ASA) score, preoperative comorbidities or postoperative pathology (all P>0.05).
Full table
Intraoperative and postoperative observation indexes
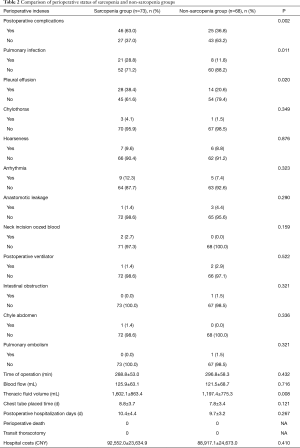
Table 2 shows the perioperative results of two groups. There were no significant differences between the groups in terms of duration of surgery and blood loss (P>0.05). We analysed data on chest fluid volume, postoperative chest tube placement time, postoperative hospitalization (days), perioperative mortality, transient open chest rate, hospitalization expenses and postoperative complications. The incidences of postoperative complications were 63.0% and 36.8% (P=0.002) in the sarcopenia and non-sarcopenia groups, respectively. In contrast, there were no significant differences between the groups in terms of chest-tube placement time, postoperative hospitalization (days), perioperative mortality, transfer rate of thoracotomy and hospitalization expenses (P>0.05). Regarding postoperative complication, the incidences of pulmonary infections and postoperative pleural effusion in the sarcopenia and non-sarcopenia groups were 28.8% and 11.8% (P=0.011) and 38.4% and 20.6% (P=0.020), respectively. There were no significant differences in the incidences of other complications (P>0.05).
Full table
Comparison of perioperative status of sarcopenia and non-sarcopenia groups
Analysis of clinical factors associated with pulmonary infection
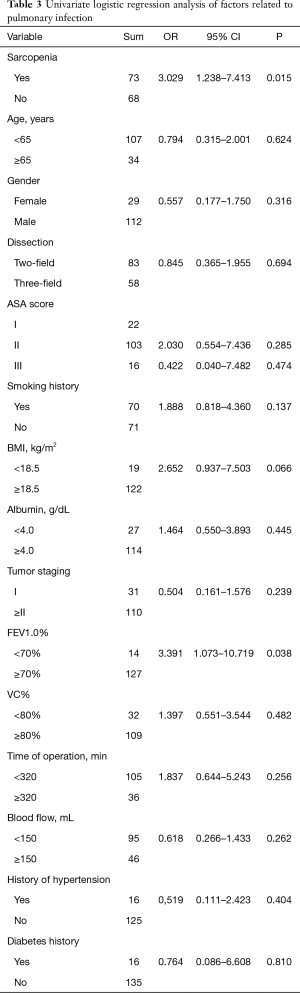
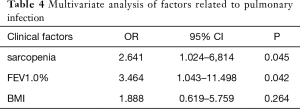
Univariate analysis revealed that sarcopenia (P=0.015) and FEV1.0% (P=0.038) were significantly associated with postoperative pulmonary complications (Table 3). To avoid omitting important clinical factors, those factors with P<0.10 in univariate analysis were included in the multivariate logistic regression analysis. Multivariate regression analysis of the above factors showed that sarcopenia and FEV1.0% were independent risk factors for pulmonary infection after MIE (P<0.05) (Table 4).
Full table
Full table
Short-term follow-up results
All patients were followed-up as outpatients by direct examinations and telephone contact. The 1-year postoperative survival rates of patients in the non-sarcopenia and sarcopenia groups were 89.4% (42/47) and 97.8% (45/46), respectively (P=0.099).
Discussion
Radical resection of oesophageal cancer is complicated, because it includes the neck, chest and abdomen. The incidence of postoperative complications is accordingly high, and postoperative respiratory complications are common after radical resection (13,14). The risk factors associated with pulmonary complications after oesophagectomy include a history of smoking, lung function, BMI and surgical methods (15-18). Respiratory complications are associated with long-term prognosis (19), and postoperative pneumonia indicates a poor prognosis (20). We therefore aimed to determine a clinical indicator that could identify patients at high risk of potential postoperative complications.
Sarcopenia, as first reported by Rosenberg in 1989, was defined as the reduction of an elderly person’s skeletal muscle (21). In 2010, the European Association on Sarcopenia in Older People (EWGSOP) redefined sarcopenia as the progressive decline of skeletal muscle area, strength and function (22). It was initially introduced as a long-term prognostic factor in patients with advanced cancer. but subsequent studies found that sarcopenia can be used as a preoperative assessment tool to predict postoperative complications (5).
Bioelectrical impedance analysis (BIA), dual-energy X-ray absorption (DXA) and magnetic resonance imaging (MRI) have previously been used to measure the SMI (23,24). However, these three technologies have shortcomings and were therefore unsuitable for the present study. For example, the reliability of BIA for determining skeletal muscle mass is affected by water content and electrode placement, and its reproducibility is poor. DXA employs ionizing radiation that may cause adverse effects. MRI is relatively expensive and time-consuming. In contrast, CT provides a relatively accurate tool for measuring skeletal muscle mass, and patients with oesophageal cancer routinely undergo preoperative CT. Therefore, we used CT in the present study to evaluate skeletal muscle mass to diagnosis sarcopenia, eliminating the requirement for additional imaging techniques.
The optimal threshold for diagnosing sarcopenia using CT is controversial. Currently, two main diagnostic criteria are used worldwide. The SMI thresholds proposed by Prado et al. (10) are widely used to assess the relationship between sarcopenia and postoperative outcomes in patients with cancers. The SMI threshold proposed by Martin et al. (25) adjusts the threshold for men according to their BMI: for a BMI is >25 kg/m2, the threshold is 53 cm2/m2, and for a BMI is ≤25 kg/m2, the threshold is 43 cm2/m2, while the SMI for women is 41 cm2/m2. Further multicentre studies are therefore required to determine the optimal criteria.
Previous studies have demonstrated a significant correlation between preoperative sarcopenia and postoperative complications of oesophageal cancer, but most of the literature comes from foreign reports, few studies have been conducted in China (5,26,27), and even fewer literature have been reported the relationship between sarcopenia and complications caused from MIE (28). All the patients in the current study underwent MIE and none underwent transit thoracotomy. In addition, previous reports have suggested that neoadjuvant therapy may have an attritional impact on body composition, leading to declines in muscle mass and strength (29). This could affect the results, and we therefore excluded patients who received neoadjuvant therapy from our study. In the present study, we found that the incidence of postoperative pulmonary infection and postoperative pleural effusion in the sarcopenia group were significantly higher compared with that of the non-sarcopenia group (P<0.05). The clinical treatment of pleural effusion is relatively simple, requiring only puncture and drainage. In contrast, the treatment of pulmonary infection is more difficult. Severe pulmonary infection may lead to hypoxemia and even respiratory failure. We therefore only analysed the independent risk factors for pulmonary infection alone. Multivariate analysis showed that sarcopenia and FEV1.0% were independent risk factors for postoperative pulmonary infection following MIE. Moreover, CT images can be used to assess whether a patient has sarcopenia before surgery, which means that the high-risk group of patients with postoperative pulmonary infection can be identified before surgery, enabling intervention to reduce postoperative complications.
Sarcopenia may affect the occurrence of pulmonary complications through different mechanisms. For example, the main manifestation of sarcopenia is the decline in skeletal muscle mass and function. Similar to other skeletal muscles, the respiratory (30) and swallowing muscles (31) are also affected by sarcopenia, particularly in patients with chronic obstructive pulmonary disease or chronic heart failure. Therefore, dysfunctions of respiration and swallowing are associated with skeletal muscle dysfunction, potentially leading to postoperative pneumonia and atelectasis. Second, the increased activity of inflammatory factors observed in patients with sarcopenia may influence pulmonary complications (32). One theory maintains that patients with skeletal muscle loss have impaired immune function and increased inflammatory activity, which may affect the development of pneumonia. However, further studies are required to identify the mechanism through which sarcopenia affects pulmonary complications, particularly pneumonia. Furthermore, preoperative skeletal muscle loss may affect long-term postoperative survival in patients with oesophageal cancer (33). The relatively short follow-up time of the present study only allowed the determination of the short-term survival rate of patients who underwent esophagectomy before December 2017. The 1-year survival rates of the non-sarcopenia and sarcopenia groups were 89.4% and 97.8%, respectively. The long-term survival rate will be determined in future studies.
Previous studies showed that the incidence of postoperative pulmonary infection was directly proportional to the degree of pulmonary dysfunction (16), and may be a cause of death during the perioperative period in patients undergoing thoracic surgery. FEV1.0% reflects obstructive airway ventilation dysfunction, and patients with low pulmonary function are more likely to have pulmonary complications after surgery (34). FEV1.0% is also closely related to the ability to cough, and effective coughing has a positive effect in terms of preventing postoperative pulmonary infections (35). The current study also found that the risk of postoperative pulmonary infection was increased in patients with an FEV1.0% <70%. It is therefore important to improve airway management and strictly avoid smoking to prevent postoperative pulmonary complications in patients with low lung function.
This study has the following limitations: First, the design of this study was retrospective, although all enrolled patients strictly met the inclusion criteria. All patients routinely underwent preoperative CT examinations, so the risk of selection bias can be ignored. Second, the optimal threshold for sarcopenia is controversial. We adopted the threshold proposed by Prado et al. which is recognized as the critical value for the diagnosis of cancer-associated cachexia. The definition of the optimal threshold needs to be confirmed by future studies. Third, the diagnosis of sarcopenia should be combined with the comprehensive judgement of skeletal muscle mass, strength and an assessment of physical fitness. The definition of sarcopenia in the present study, which refers to other published reports, was based on skeletal muscle mass alone. The validity of this criterion thus requires confirmation in further studies.
Despite these limitations, the results of the present study suggest that sarcopenia may be a new and independent predictor of pulmonary complications after radical resection of oesophageal cancer. Identifying patients with preoperative sarcopenia helps provide clinicians with useful clinical information to aid treatment decisions. Furthermore, evidence indicates that exercise and nutritional support may improve complications and the prognosis in patients with sarcopenia (36-38). Therefore, appropriate perioperative management methods can be adopted, such as preoperative respiratory exercise, protein supplementation and other methods that improve the condition of skeletal muscles and prevent postoperative complications.
Conclusions
In summary, sarcopenia diagnosed by preoperative CT imaging may be an independent risk factor for postoperative pulmonary infection in patients with oesophageal cancer. However, further studies are needed to determine the value of sarcopenia for assessing long-term outcomes following MIE.
Acknowledgments
We thank Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Li Y, Sun H. Progress and prospect of enhanced recovery after surgery for esophageal cancer Chin J Thoracic Surg 2017;4:140-8. (in Chinese).
- Takeuchi H, Miyata H, Gotoh M, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nation wide web-based data base. Ann Surg 2014;260:259-66. [Crossref] [PubMed]
- Lagarde SM, Reitsma JB, Maris AK, et al. Preoperative prediction of the occurrence and severity of complications after esophagectomy for cancer with use of a nomogram. Ann Thorac Surg 2008;85:1938-45. [Crossref] [PubMed]
- Pichard C, Kyle UG. Body composition measurements during wasting diseases. Curr Opin Clin Nutr Metab Care 1998;1:357-61. [Crossref] [PubMed]
- Nishigori T, Okabe H, Tanaka E, et al. Sarcopenia as a predictor of pulmonary complications after esophagectomy for thoracic esophageal cancer. J Surg Oncol 2016;113:678-84. [Crossref] [PubMed]
- Harimoto N, Yoshizumi T, Shimokawa M, et al. Sarcopenia is a poor prognostic factor following hepatic resection in patients aged 70 years and older with hepatocellular carcinoma. Hepatol Res 2016;46:1247-55. [Crossref] [PubMed]
- Smith AB, Deal AM, Yu H, et al. Sarcopenia as a predictor of complications and survival following radical cystectomy. J Urol 2014;191:1714-20. [Crossref] [PubMed]
- Sur MD, Namm JP, Hemmerich JA, et al. Radiographic sarcopenia and self-reported exhaustion independently predict NSQIP serious complications after pancreaticoduodenectomy in older adults. Ann Surg Oncol 2015;22:3897-904. [Crossref] [PubMed]
- Van Vledder MG, Levolger S, Ayez N, et al. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg 2012;99:550-7. [Crossref] [PubMed]
- Prado CM, Lieffers JR, McCargar L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 2008;9:629-35. [Crossref] [PubMed]
- Low DE, Alderson D, Cecconello I, et al. International Consensus on Standardization of Data Collection for Complications Associated with Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286-94. [Crossref] [PubMed]
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien Dindo classification of surgical complications. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Sakatoku Y, Fukaya M, Miyata K, et al. Clinical value of a prophylactic minitracheostomy after esophagectomy: analysis in patients at high risk postoperative for pulmonary complications. BMC Surg 2017;17:120. [Crossref] [PubMed]
- Yoshida N, Watanabe M, Baba Y, et al. Risk factors for pulmonary complications after esophagectomy for esophageal cancer. Surg Today 2014;44:526-32. [Crossref] [PubMed]
- Ferguson MK, Celauro AD, Prachand V. Prediction of major pulmonary complications after esophagectomy. Ann Thorac Surg 2011;91:1494-500; discussion 1500-491.
- Yoshida N, Baba Y, Hiyoshi Y, et al. Duration of Smoking Cessation And Postoperative Morbidity After Esophagectomy for Esophageal Cancer: How Long Should Patients Stop Smoking Before Surgery? World J Surg 2016;40:142-7. [Crossref] [PubMed]
- Miao L, Chen H, Xiang J, et al. A high body mass index in esophageal cancer patients is not associated with adverse outcomes following esophagectomy. J Cancer Res Clin Oncol 2015;141:941-50. [Crossref] [PubMed]
- Xiong WL, Li R, Lei HK, et al. Comparison of outcomes between Minimally invasive oesophagectomy and open oesophagectomy for oesophageal cancer. ANZ J Surg 2017;87:165-170. [Crossref] [PubMed]
- Ichikawa H, Kosugi SI, Kanda T, et al. Surgical and long-term outcomes following oesophagectomy in oesophageal cancer patients with comorbidity. Int J Surg 2016;36:212-8. [Crossref] [PubMed]
- Booka E, Takeuchi H, Nishi T, et al. The Impact of Postoperative Complications on Survivals After Esophagectomy for Esophageal Cancer. Medicine (Baltimore) 2015;94:e1369. [Crossref] [PubMed]
- Rosenberg I. Summary comments: epidemiological and methodological problems in determining nutritional status of older persons. Am J Clin Nutr 1989;50:1231-3. [Crossref]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412-23. [Crossref] [PubMed]
- Jin JX. Progress on the diagnosis and management of sarcopenia Chin J Geriatr 2015;34:1154-6. (in Chinese).
- Yan C, Tang G, Cheng X. State of the art of quantitative measurement of sarcopenia Chin J Osteoporos 2018;24:814-9. (in Chinese).
- Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539-47. [Crossref] [PubMed]
- Soma D, Kawamura Y, Yamashita S, et al. Sarcopenia, the depletion of muscle mass, an independent predictor of respiratory complications after oncological esophagectomy. Dis Esophagus 2019. [Crossref] [PubMed]
- Makiura D, Ono R, Inoue J, et al. Preoperative sarcopenia is a predictor of postoperative pulmonary complications in esophageal cancer following esophagectomy: A retrospective cohort study. J Geriatr Oncol 2016;7:430-6. [Crossref] [PubMed]
- Boshier PR, Heneghan R, Markar SR, et al. Assessment of body composition and sarcopenia in patients with esophageal cancer: a systematic review and meta-analysis. Dis Esophagus 2018. [Crossref] [PubMed]
- Guinan EM, Doyle SL, Bennett AE, et al. Sarcopenia during neoadjuvant therapy for oesophageal cancer: characterizing the impact on muscle strength and physical performance. Support Care Cancer 2018;26:1569-76. [PubMed]
- Bahat G, Tufan A, Ozkaya H, et al. Relation between hand grip strength, respiratory muscle strength and spirometric measures in male nursing home residents. Aging Male 2014;17:136-40. [Crossref] [PubMed]
- Wakabayashi H, Sakuma K. Rehabilitation nutrition for sarcopenia with disability: A combination of both rehabilitation and nutrition care management. J Cachexia Sarcopenia Muscle 2014;5:269-77. [Crossref] [PubMed]
- Kooguchi K, Kobayashi A, Kitamura Y, et al. Elevated expression of inducible nitric oxide synthase and inflammatory cytokines in the alveolar macrophages after esophagectomy. Crit Care Med 2002;30:71-6. [Crossref] [PubMed]
- Deng HY, Zha P, Peng L, et al. Preoperative sarcopenia is a predictor of poor prognosis of esophageal cancer after esophagectomy: a comprehensive systematic review and meta-analysis. Dis Esophagus 2019. [Crossref] [PubMed]
- Liu LX, Hu ZJ, Zzhao C. Correlations of Preoperative Pulmonary Function Tests for Esophageal Cancer to Postoperative Acute Respiratory Distress Syndrome. Ai Zheng 2006;25:335-8. [PubMed]
- Lu CW, Chang YK, Chang HH, et al. Fracture risk after bariatric surgery: a 12-year nationwide cohort study. Medicine (Baltimore) 2015;94:e2087. [Crossref] [PubMed]
- Beaudart C, Dawson A, Shaw SC, et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporos Int 2017;28:1817-33. [Crossref] [PubMed]
- Kraft M, Kraft K, Gärtner S, et al. L-Carnitine-supplementation in advanced pancreatic cancer (CARPAN)-a randomized multicentre trial. Nutr J 2012;11:52. [Crossref] [PubMed]
- Moran J, Guinan E, Mccormick P, et al. The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: a systematic review and meta-analysis. Surgery 2016;160:1189-201. [Crossref] [PubMed]