
Clinical relevance of chronic respiratory disease in Korean patients with pulmonary thromboembolism
Introduction
Venous thromboembolism (VTE), which encompasses deep vein thrombosis (DVT) and pulmonary thromboembolism (PTE), can be categorized into provoked or unprovoked according to the presence or absence of a disease or condition that predispose to VTE (1,2). Provoking risk factors vary, ranging from major transient risk factors, including recent major surgery, to persistent risk factors, such as active cancer (3). In addition, some PTE patients have various comorbid conditions, which may or may not contribute to the development of VTE (2). However, it is difficult to determine whether the mere presence of comorbidities is sufficient to develop VTE or only coexisting conditions. Evidence suggests that chronic inflammatory conditions, such as inflammatory bowel disease (4,5), autoimmune disease (6), and chronic infections (7), can serve as persistent provoking factors (3).
There is accumulating evidence regarding the clinical relevance of chronic respiratory diseases (CRDs), including chronic obstructive pulmonary disease (COPD), asthma, and idiopathic pulmonary fibrosis (IPF), in patients with PTE. IPF patients have a two-fold higher risk for VTE than the general population (8) and can experience clinical deterioration due to PTE (9). PTE can also cause acute exacerbation of COPD (10). Moreover, the risk for developing PTE is significantly increased in patients with asthma compared with the general population (11). Along with cancer and heart failure, CRD constitutes components determining the Pulmonary Embolism Severity Index (PESI) (12) or Simplified PESI score (13), the most widely adopted clinical prediction rule of PTE to predict the short-term prognosis of PTE. Therefore, coexisting CRD in PTE is presumed to play a role in predicting short-term prognosis. However, data regarding clinical and radiological features of PTE patients with CRD are lacking. We hypothesized that patients with PTE and concomitant CRD exhibit distinctive clinico-radiologic features. Thus, we investigated the clinical characteristics and laboratory and computed tomographic (CT) findings in patients with PTE and coexisting CRD.
Methods
Study design
The present retrospective study was performed at the Kyungpook National University Hospital (KNUH), a tertiary referral center located in Daegu, South Korea. Data from consecutive hospitalized patients with PTE diagnosed via CT angiography between March 2003 and December 2017 were collected. Patients with suspected chronic thromboembolic pulmonary hypertension were not included. CRD included COPD, asthma, bronchiectasis, interstitial lung disease, bronchial anthracofibrosis, and tuberculosis-destroyed lung. The presence or absence of coexisting CRDs was determined by consensus of two chest physicians (H Park and SI Cha) based on medical records. The diagnosis of COPD and asthma was adopted from a review of patient medical records. Bronchial anthracofibrosis was diagnosed based on CT findings that fulfilled the following criteria: bronchostenosis that caused smooth narrowing of multiple lobar or segmental bronchi; calcified or non-calcified lymph node enlargement adjacent to narrowed bronchi; and these two criteria which could not be attributed to other causes (14). The diagnosis of tuberculosis-destroyed lung was based on CT finding of lung parenchymal destruction with lung volume loss and/or bronchiectatic changes in more than one lobe in patients with previous pulmonary tuberculosis and with no evidence of active tuberculosis, such as culture for respiratory specimens (15). All PTE patients were divided into those with CRD (CRD group) and without CRD (control group); clinical characteristics, blood biomarkers, and CT findings were compared between the two groups. This study was approved by the Institutional Review Board of the KNUH. Given the retrospective nature of the present study and the use of anonymized patient data, requirements for informed written consent were waived.
Data collection
Demographic information and data regarding presenting manifestations, vital signs, risk factors for VTE, and comorbid conditions, were reviewed. Unprovoked PTE was defined as the absence of provoking risk factors, including trauma, surgery, cancer, pregnancy and puerperium within 3 months of the event, and immobilization (bed rest for most of the day for ≥3 consecutive days) within 1 month of the event (10). The PESI score was calculated retrospectively (13). Outcome variables, including PTE-related in-hospital mortality, adverse outcomes, and VTE recurrence, were examined. PTE-related in-hospital death was defined as an in-hospital death that met one of the following criteria: objective evidence of death directly caused by PTE; and death that could not be attributed to other causes and in which PTE could not be excluded (16). An adverse outcome was defined as a PTE-related in-hospital death or PTE resulting in a serious clinical condition requiring intensive care treatment, such as inotropic support and mechanical ventilation, impending respiratory failure or refractory hypoxia, cardiopulmonary resuscitation, or secondary thrombolysis (16). According to the 2014 European Society of Cardiology (ESC) guidelines (2), the severity of PTE in each patient was classified into four tiers: high, intermediate-high, intermediate-low, and low risk. Blood laboratory data included biomarkers, such as N-terminal-pro-B-type natriuretic peptide (NT-proBNP) and troponin I, and inflammatory markers, including erythrocyte sedimentation rate, C-reactive protein, and procalcitonin.
Radiological evaluation
PTE was diagnosed on CT images as a sharply delineated filling defect in a pulmonary artery in at least two consecutive image sections and that was located either centrally within the vessel or formed acute angles with the arterial wall (17). The largest pulmonary artery involved by pulmonary thromboemboli was classified as follows: pulmonary trunk or right atrium, pulmonary (right or left pulmonary artery), interlobar or lobar, segmental, and subsegmental. Central PTE denoted thromboemboli located in the right or left pulmonary artery or more proximal sites. Similar to a previous study (16), the diameters of the right ventricle (RV) and left ventricle (LV) were measured at their widest points between the inner surface of the free wall and the surface of the interventricular septum; RV/LV diameter ratios were calculated; and RV dilation was defined as an RV/LV diameter ratio ≥1. The diameters of the pulmonary trunk and ascending aorta were measured at the level of pulmonary trunk bifurcation to obtain the pulmonary trunk-to-ascending aorta ratio (PA/AA ratio) (18,19); pulmonary artery enlargement was defined as having PA/AA ratio >1. Pulmonary infarction was defined as the presence of a peripheral consolidation in the region of segmental or subsegmental pulmonary emboli (20). Similar to a previous study (21), when a soft tissue density was visualized in distinctly narrowed, totally obstructed, or dilated pulmonary arteries or their branches from axial images, in-situ pulmonary artery thrombosis was diagnosed, from which patients with coexisting DVT on CT venography or ultrasonography were excluded.
Statistical analysis
Data are expressed as mean with standard deviation for continuous variables and as numbers with percentages for categorical variables. Continuous variables between groups were compared using Student’s t-test, and categorical variables were compared using chi-squared test or Fisher’s exact test. Multiple logistic regression analysis was used to identify clinical factors associated with CRD in PTE patients and predictors of PTE-related in-hospital mortality. The Hosmer-Lemeshow test was used as a goodness-of-fit test to assess the fit of logistic regression models. P value <0.05 were considered to be statistically significant. Statistical analysis was performed using SPSS version 23.0 (IBM Corporation, Armonk, NY, USA) for Windows (Microsoft Corporation, Redmond, WA, USA).
Results
Clinical characteristics of PTE patients with CRD
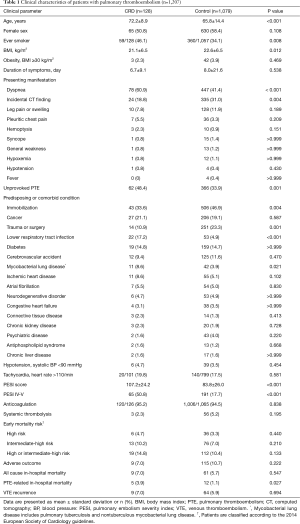
A total of 1,216 hospitalized PTE patients were identified during the study period; however, nine without available enhanced CT scans were excluded. Ultimately, 1,207 PTE patients were included in this study and then divided into PTE patients with CRD [n=128 (11%)] and without CRD (n=1,079) (Figure 1). The most common CRD was COPD [41 (32%)], followed by bronchial anthracofibrosis [32 (25%)] and tuberculosis-destroyed lung [21 (16%)] (Figure 2).


The clinical characteristics of the CRD group are presented in Table 1. As expected, patients with CRD were significantly older (72.2±8.9 vs. 65.8±14.4 years, P<0.001) and had a significantly higher proportion of ever-smokers [59/128 (46%) vs. 360/1,057 (34%), P=0.008] than those without CRD. The CRD group more frequently presented with dyspnea [78 (61%) vs. 447 (41%), P<0.001]; however, pulmonary thromboemboli were less frequently incidentally detected on CT in the CRD group than in the control group [24 (19%) vs. 335 (31%), P=0.004]. The CRD group had a significantly higher percentage of unprovoked PTE [62 (48%) vs. 366 (34%), P=0.001] but significantly lower rates of immobilization [43 (34%) vs. 506 (47%), P=0.004] and trauma or surgery [14 (11%) vs. 251 (23%), P=0.001] than the control group. LRTI [22 (17%) vs. 53 (5%), P<0.001] and mycobacterial lung disease [11 (9%) vs. 42 (4%), P=0.021] more frequently occurred in the CRD group than in the control group. The CRD group had a significantly higher incidence of PESI class IV–V [65 (51%) vs. 191 (18%), P<0.001] than the control group. In contrast, the proportion of patients with high or intermediate-high risk according to the 2014 ESC guidelines and the frequency of adverse outcomes did not differ between the two groups. However, the CRD group experienced significantly higher PTE-related in-hospital mortality than the control group [5 (4%) vs. 12 (1%), P=0.027]. The rate of VTE recurrence was not significantly different between the two groups.
Full table
Laboratory and CT findings in PTE patients with CRD
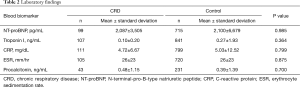
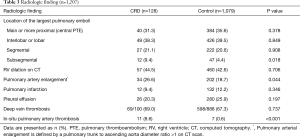
The blood levels of NT-proBNP, troponin I, and inflammatory markers were not significantly different between the CRD and control groups (Table 2). CT findings are summarized in Table 3. The distribution of the location of the largest pulmonary emboli was not different. The incidence of RV dilation on CT was similar between the two groups; however, pulmonary artery enlargement was significantly more common in the CRD group than in the control group [34 (27%) vs. 202 (19%), P=0.044]. In-situ pulmonary artery thrombosis, not pulmonary embolism, was also significantly more commonly found in the CRD group than in the control group [12 (9%) vs. 9 (1%), P<0.001]. The most common underlying condition of in-situ pulmonary artery thrombosis was tuberculosis-destroyed lung (n=10), followed by lung resection (n=4) and adhesive obliteration (n=4). The frequency of pulmonary infarction was not significantly different between the two groups.
Full table
Full table
Clinical parameters related to CRD in patients with PTE
Variables with P value <0.05 in univariate analysis, including unprovoked PTE, ever-smoker, dyspnea, incidental CT finding, immobilization, trauma or surgery, LRTI, mycobacterial lung disease, pulmonary artery enlargement, in-situ pulmonary artery thrombosis, and PESI class IV–V, were selected as candidate variables for multivariate analysis to investigate the clinical and radiological parameters associated with CRD in PTE patients. Multivariate analysis (Hosmer-Lemeshow test, P=0.820) demonstrated that unprovoked PTE [odds ratio (OR) 1.99, 95% confidence interval (CI): 1.29–3.05, P=0.002], dyspnea (OR 1.54, 95% CI: 1.11–2.34, P=0.041), LRTI (OR 3.90, 95% CI: 2.13–7.14, P<0.001), PESI class IV–V (OR 5.24, 95% CI: 3.43–8.00, P<0.001), in-situ pulmonary artery thrombosis (OR 10.62, 95% CI: 3.71–30.45, P<0.001), and pulmonary artery enlargement (OR 1.65, 95% CI: 3.71–30.45, P<0.001) were independent clinical factors associated with CRD in PTE patients (Table 4).

Full table
Predictors of PTE-related in-hospital death
As noted above, the CRD group experienced a higher PTE-related in-hospital mortality than the control group. Age, sex, and parameters with P value <0.05 in univariate analysis, including CRD, cerebrovascular accident, central PTE, and RV dilation on CT, were chosen as candidate variables to examine predictors of PTE-related in-hospital death (Table 5). The multivariate analysis (Hosmer-Lemeshow test, P=0.701) confirmed that CRD (OR 3.96, 95% CI: 1.32–11.88, P=0.014) was an independent predictor of PTE-related in-hospital mortality along with cerebrovascular accident, hypotension, and RV dilation on CT.

Full table
Discussion
In the present study, 11% of the patients with PTE had concomitant CRD. Compared with non-CRD patients, PTE patients with CRD were more likely to be older with a higher proportion of ever-smokers, present with dyspnea, and be associated with LRTI. PTE patients with CRD demonstrated a higher PESI score and a higher incidence of PESI class IV–V than those without CRD. In addition, the PTE patients with CRD had a higher PTE-related in-hospital mortality rate than those without CRD; this association was confirmed through multivariate analysis. Multivariate analysis revealed that unprovoked PTE, dyspnea, LRTI, PESI class IV–V, in-situ pulmonary artery thrombosis, and pulmonary artery enlargement were associated with PTE in CRD patients.
Mechanisms how CRD predisposes to the development of VTE have been proposed. First, several studies reported that CRD, such as COPD (22,23) or IPF itself (24), is associated with a procoagulant state. Second, immobility caused by CRD can contribute to VTE pathogenesis and increase mortality rates during hospitalization and 30 days after VTE diagnosis (25). Third, various comorbid conditions of CRD, such as heart failure, ischemic heart disease, and chronic kidney disease, increases the risk for VTE (25). Finally, as reported above, PTE patients with CRD were more often associated with LRTI than non-CRD patients. LRTIs, such as pneumonia, have been reported to be correlated with the development of VTE (26), thus further increasing the risk for PTE. Compared to the non-CRD patients, the CRD patients were less frequently associated with immobilization and trauma or surgery in the present study. This finding contradicts the concept that immobility caused by CRD contributes to VTE. We speculate that this discrepancy may be due to the difference in the definitions between immobilization and immobility: in this study, immobilization, a more severe form, was defined as bed rest for most of the day for ≥3 consecutive days.
Short-term prognostic factors of PTE include clinical prediction rules, such as the PESI score, the levels of blood biomarkers, such as troponin I and NT-proBNP, and echocardiographic RV dysfunction or RV dilation on CT (2). The presence of CRD can directly increase the PESI score (12) by adding 10 points and indirectly by adding points, for example, due to age, with a mean difference of 6 in the present study. Thus, the CRD group had a significantly higher PESI score and a higher incidence of PESI class IV–V than the control group. On the other hand, the blood levels of biomarkers and the incidence of RV dilation on CT were not significantly different between the CRD and control groups. Consequently, the proportion of patients with high or intermediate-high risk according to 2014 ESC guidelines did not differ between the two groups in the present study.
The CT characteristics of PTE in patients with CRD are explored below. First, the frequency of in-situ pulmonary artery thrombosis was significantly higher in the CRD group. In the present study, the most common underlying condition of in-situ pulmonary artery thrombosis was tuberculosis-destroyed lung, which led to a higher incidence of in-situ pulmonary artery thrombosis in the CRD group than in the control group. This can be explained by the fact that South Korea has an intermediate prevalence of active tuberculosis, and chest physicians commonly encounter patients with tuberculosis-destroyed lung (21). Second, pulmonary infarction was less frequently observed in the CRD group than in the control group, despite the lack of statistical significance. The classical concept of pulmonary infarction is that pulmonary infarction occurs more commonly in patients with underlying conditions such as heart disease, chronic lung disease, and malignancy, compared with patients without these comorbidities (20). A previous study reported that the incidence of chronic lung disease did not differ between patients with and without pulmonary infarction (20). A more recent study demonstrated that younger PTE patients without cardiopulmonary comorbidities were at highest risk for pulmonary infarction (27). They proposed that older PTE patients have more developed bronchopulmonary collateral vessels due to chronic cardiopulmonary disease, which provides protection against pulmonary infarction after PTE. Finally, in the present study, the frequency of pulmonary artery enlargement was significantly higher in the CRD group than in the control group. This can be explained by reported findings of pulmonary artery enlargement in chronic lung diseases (28), including COPD (29), bronchial anthracofibrosis (14), tuberculosis-destroyed lung (30), and IPF (31,32).
Among the variables associated with treatment outcome, the CRD group demonstrated a significantly higher PTE-related in-hospital mortality rate than the control group. This finding was confirmed by multivariate analysis. A population-based study demonstrated that COPD patients were more likely to experience a complicated course and die in hospital and within 30 days of VTE diagnosis (25). Data from a registry suggested that COPD patients also have a worse three-month mortality rate than non-COPD patients (33). Based on these data, underlying CRD may unfavorably affect the short-term prognosis of PTE. PTE-related in-hospital mortality and adverse outcomes are endpoints that both signify the short-term prognosis of PTE. However, whereas PTE patients with CRD demonstrated a higher PTE-related in-hospital mortality than those without CRD, the incidence of adverse outcomes did not differ between the two groups. This discrepancy can be explained, in part, by the fact that the severity of concomitant CRDs varied from a mild to severe form, which subsequently affected cardiopulmonary reserve to a very negligible extent in some PTE individuals or to a greater degree in others.
Several limitations of this investigation should be addressed. First, selection bias was inevitable, given that this was a retrospective study performed in a single center. Furthermore, because the study population was restricted to hospitalized PTE patients, CRD might less often complicate all-comers with PTE. Second, because the presence of concomitant CRD was identified through review of medical records, the possibility that the incidence of CRD was underestimated could not be excluded. Third, patients with various CRDs, such as COPD, were treated as a single group; however, diverse characteristics of each CRD may offset one another. Fourth, the all-cause in-hospital mortality reported in the present study was lower than that reported in previous studies. Thus, the possibility that PTE patients who died were excluded from this study should be considered. Finally, missing laboratory data, such as NT-proBNP levels, may have influenced the results.
In conclusion, patients with PTE and concomitant CRD were more likely to present with dyspnea in elderly individuals with a higher proportion of ever-smoker and less likely to be incidentally detected on CT, compared with PTE patients without CRD. LRTIs, such as pneumonia, more frequently accompanied the development of PTE in CRD patients. These patients were characterized by a high PESI score and in particular, in Koreans, a higher prevalence of in-situ pulmonary artery thrombosis. The presence of CRD may influence PTE-related in-hospital mortality in patients with PTE.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Review Board of the KNUH. Given the retrospective nature of the present study and the use of anonymized patient data, requirements for informed written consent were waived.
References
- Kearon C. Duration of anticoagulation for venous thromboembolism. J Thromb Thrombolysis 2001;12:59-65. [Crossref] [PubMed]
- Kearon C, Ageno W, Cannegieter SC, et al. Categorization of patients as having provoked or unprovoked venous thromboembolism: guidance from the SSC of ISTH. J Thromb Haemost 2016;14:1480-3. [Crossref] [PubMed]
- Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014;35:3033-69, 3069a-k.
- Nguyen GC, Bernstein CN, Bitton A, et al. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology 2014;146:835-48.e6. [Crossref] [PubMed]
- Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet 2010;375:657-63. [Crossref] [PubMed]
- Holmqvist ME, Neovius M, Eriksson J, et al. Risk of venous thromboembolism in patients with rheumatoid arthritis and association with disease duration and hospitalization. JAMA 2012;308:1350-6. [Crossref] [PubMed]
- Tichelaar YI, Kluin-Nelemans HJ, Meijer K. Infections and inflammatory diseases as risk factors for venous thrombosis. A systematic review. Thromb Haemost 2012;107:827-37. [Crossref] [PubMed]
- Ungprasert P, Wijarnpreecha K, Thongprayoon C. Risk of venous thromboembolism in patients with bullous pemphigoid: A systematic review and meta-analysis. Indian J Dermatol Venereol Leprol 2018;84:22-6. [Crossref] [PubMed]
- Panos RJ, Mortenson RL, Niccoli SA, et al. Clinical deterioration in patients with idiopathic pulmonary fibrosis: causes and assessment. Am J Med 1990;88:396-404. [Crossref] [PubMed]
- Choi KJ, Cha SI, Shin KM, et al. Prevalence and predictors of pulmonary embolism in Korean patients with exacerbation of chronic obstructive pulmonary disease. Respiration 2013;85:203-9. [Crossref] [PubMed]
- Chung WS, Lin CL, Ho FM, et al. Asthma increases pulmonary thromboembolism risk: a nationwide population cohort study. Eur Respir J 2014;43:801-7. [Crossref] [PubMed]
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005;172:1041-6. [Crossref] [PubMed]
- Jiménez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010;170:1383-9. [Crossref] [PubMed]
- Kim H, Cha SI, Shin KM, et al. Clinical relevance of bronchial anthracofibrosis in patients with chronic obstructive pulmonary disease exacerbation. Tuberc Respir Dis (Seoul) 2014;77:124-31. [Crossref] [PubMed]
- Rhee CK, Yoo KH, Lee JH, et al. Clinical characteristics of patients with tuberculosis-destroyed lung. Int J Tuberc Lung Dis 2013;17:67-75. [Crossref] [PubMed]
- Choi KJ, Cha SI, Shin KM, et al. Prognostic implications of computed tomographic right ventricular dilation in patients with acute pulmonary embolism. Thromb Res 2014;133:182-6. [Crossref] [PubMed]
- Gladish GW, Choe DH, Marom EM, et al. Incidental pulmonary emboli in oncology patients: prevalence, CT evaluation, and natural history. Radiology 2006;240:246-55. [Crossref] [PubMed]
- Devaraj A, Wells AU, Meister MG, et al. Detection of pulmonary hypertension with multidetector CT and echocardiography alone and in combination. Radiology 2010;254:609-16. [Crossref] [PubMed]
- Edwards PD, Bull RK, Coulden R. CT measurement of main pulmonary artery diameter. Br J Radiol 1998;71:1018-20. [Crossref] [PubMed]
- Cha SI, Shin KM, Lee J, et al. Clinical relevance of pulmonary infarction in patients with pulmonary embolism. Thromb Res 2012;130:e1-5. [Crossref] [PubMed]
- Cha SI, Choi KJ, Shin KM, et al. Clinical characteristics of in-situ pulmonary artery thrombosis in Korea. Blood Coagul Fibrinolysis 2015;26:903-7. [Crossref] [PubMed]
- Tapson VF. The role of smoking in coagulation and thromboembolism in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005;2:71-7. [Crossref] [PubMed]
- Vaidyula VR, Criner GJ, Grabianowski C, et al. Circulating tissue factor procoagulant activity is elevated in stable moderate to severe chronic obstructive pulmonary disease. Thromb Res 2009;124:259-61. [Crossref] [PubMed]
- Crooks MG, Hart SP. Coagulation and anticoagulation in idiopathic pulmonary fibrosis. Eur Respir Rev 2015;24:392-9. [Crossref] [PubMed]
- Piazza G, Goldhaber SZ, Kroll A, et al. Venous thromboembolism in patients with chronic obstructive pulmonary disease. Am J Med 2012;125:1010-8. [Crossref] [PubMed]
- Clayton TC, Gaskin M, Meade TW. Recent respiratory infection and risk of venous thromboembolism: case-control study through a general practice database. Int J Epidemiol 2011;40:819-27. [Crossref] [PubMed]
- Islam M, Filopei J, Frank M, et al. Pulmonary infarction secondary to pulmonary embolism: An evolving paradigm. Respirology 2018;23:866-72. [Crossref] [PubMed]
- Grosse C, Grosse A. CT findings in diseases associated with pulmonary hypertension: a current review. Radiographics 2010;30:1753-77. [Crossref] [PubMed]
- Wells JM, Washko GR, Han MK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med 2012;367:913-21. [Crossref] [PubMed]
- Lee SW, Shim SS, Ryu YJ, et al. Tuberculous-destroyed lung: cardiovascular CT findings and prognostic imaging factors. Clin Imaging 2013;37:1000-5. [Crossref] [PubMed]
- Yagi M, Taniguchi H, Kondoh Y, et al. CT-determined pulmonary artery to aorta ratio as a predictor of elevated pulmonary artery pressure and survival in idiopathic pulmonary fibrosis. Respirology 2017;22:1393-9. [Crossref] [PubMed]
- Furukawa T, Kondoh Y, Taniguchi H, et al. A scoring system to predict the elevation of mean pulmonary arterial pressure in idiopathic pulmonary fibrosis. Eur Respir J 2018;51:1701311. [Crossref] [PubMed]
- Bertoletti L, Quenet S, Mismetti P, et al. Clinical presentation and outcome of venous thromboembolism in COPD. Eur Respir J 2012;39:862-8. [Crossref] [PubMed]


