
Comparing attenuations of malignant and benign solitary pulmonary nodule using semi-automated region of interest selection on contrast-enhanced CT
Introduction
Radiologically indeterminate solitary pulmonary nodules (SPN) on chest CT images often pose a challenge to radiologists in establishing a benign or malignant diagnosis. In a similar respect, SPN found on clinical routine practice settings, regardless of their frequency, are never be underestimated; A recent study demonstrated that 12.4% of the SPNs detected in routine chest CT was lung cancer (1). For this reason, there have been many studies on evaluating malignant characteristics of SPN (2-7). It is well known that CT diagnostic analysis for predicting likelihood of malignancy of SPN is based on the lesion’s size, growth, morphology and attenuation. Notable studies by Swensen et al. (2,3) showed that SPN’s net enhancement of 15 HU or more correlates with malignancy.
In routine clinical settings, the CT density measurement is usually measured manually by drawing region of interest (ROI) in most of soft tissue of the lesion. However, manual analysis falls into subjective interpretation bias and such measurement may not truly reflect the entire lesion’s enhancement patterns. Also, SPN with various sizes, margins and internal characteristics such as calcification, fat, air and fluid could further make the measurement a difficult process. The present study included the entire SPN for measuring ROI by semi-automated method with preset range of threshold attenuations.
The goal of the present study was to measure attenuation of various SPN beyond the scope of well-known SPN criteria using semi-automated ROI selection and subsequently determining sensitivity, specificity and accuracy for predicting malignancy.
Methods
This retrospective study was approved by the institutional review board of our institution with a waiver of the requirement for informed consent of the patients.
Study population
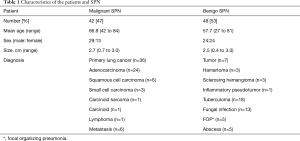
A total of 115 patients with SPN detected on initial chest CT scans at our institution during 2 years were retrospectively reviewed. Peripheral lung nodules, compatible with lung-RAD, or Fleischer’s society definition for SPN were chosen in this study. Finally, CT scans of ninety patients [56 men (63±12 years) and 34 women (62±11 years)] were reviewed by three thoracic radiologists (Y Choi, BM Gil and MH Chung, each with 2, 5 and 25 years of experience in thoracic imaging, respectively) via Picture Archiving and Communication System (PACS). SPN were defined as variable-sized (0.4–3.0 cm) solitary nodules of any shape located within lung parenchyma. The patient demographics findings are summarized in Table 1.
Full table
Image acquisition and semi-automated CT ROI selection
SPN with various shapes and internal contents (rounded, lobulated and cavitary) on CT scans were included. Nodules containing benign-looking calcifications were excluded while those with stippled and peripheral small calcifications, uncertain low density areas or even small and large air cavities were included because any attenuation above 200 HU and below −100 HU (calcification, bony component and air) were automatically eliminated and only the remaining soft tissue components including fat and necrotic densities were measured by our method.
A 64-slice MDCT scanner (SOMATOM Sensation 64, Siemens Medical Solution, Forchheim, Germany) was used in 54 patients and a 128-slice MDCT (Discovery CT 750 HD, GE Medical Systems, Waukesha, WI, USA) was used in the rest of 36 patients with SPN. All CT scans were obtained with a 3-mm collimation at 3-mm intervals using a tube voltage of 120 kV and tube current of 130 mA covering the apices of the lungs to the pleural recesses. All chest MDCT images were reconstructed with the lung window settings (width, 1,000 to 1,500 HU; center, −700 HU) and with the mediastinum settings (width, 350 HU; center, 50 HU). Post-enhanced scan was obtained 70 seconds after contrast media (Ultravist, Shering, Berlin, Germany) injection; 120 mL of contrast media was injected at a rate of 2.0 mL/s using a power injector (MEDRAD, Stellant, Bayer, Leverkusen, Germany) without subsequent flushing with normal saline. All patients were in supine position at full inspiration during all CT scans, which approximately took 3 minutes. All reconstructed MDCT images were transferred to workstations for quantitative assessment.
The transferred raw date images were analyzed by the three thoracic radiologists at the dedicated two workstations with software (GE AW area histogram, General Electrics, Fairfield, CT, USA; and Siemens, Lung Parenchyma Analysis, Siemens Medical Solution, Forchheim, Germany) that are programmed with algorithms for the automatic ROI assessment.
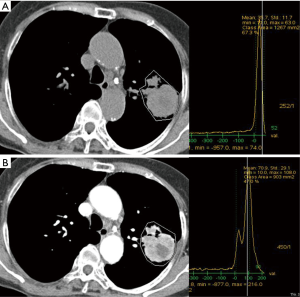
The ROIs of non-enhanced and contrast-enhanced CT images were roughly drawn peripherally around SPN, because surrounding lung airs were automatically eliminated. A predetermined range of densities (minimum: −100 HU, maximum: 200 HU) was entered into the software in order to eliminate calcifications and air allowing selective measurement of soft tissue densities. The ROIs included the entire SPN regardless of its shape or internal contents (Figure 1).
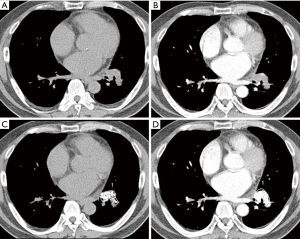
The procedure for semi-automated ROI selection of SPN using Siemens and GE AW software included freehand ROI drawn on entire SPN. The pre-enhanced attenuation (PreEA) is the ROI value of SPN on the non-enhanced CT image and the enhanced attenuation (EA) is the ROI value of SPN on the contrast-enhanced CT image. Net-enhanced attenuation (NetEA) is the value of EA minus that of PreEA.
Statistical analysis
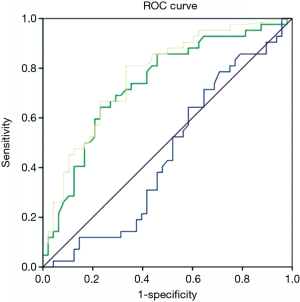
Statistical analyses were performed using commercially available SPSS software for Windows (version 19.0; IBM Corp., Armonk, NY, USA). Continuous variables were presented as mean ± standard deviation (SD). Mann-Whitney U test were used for comparing statistically significant differences in mean attenuations of PreEA, EA and NetEA between malignant and benign SPN. A P value <0.05 was considered to be significant. We computed a receiving operator characteristic (ROC) curve to derive sensitivity, specificity, and accuracy for predicting SPN’s malignancy (Figure 2).
Results
There were 84 pathologically proven cases, 55 CT-guided biopsy and 29 video-assisted thoracoscopic surgery (VATS) while the rest 6 cases were diagnosed by clinical observation such that no interval change of the SPN over two years of follow-up CT scans was considered benign. The prevalence of malignancy was 47% (42/90). The cell type of malignant tumors are as follows; 24 adenocarcinoma, 6 squamous cell carcinoma, 3 small cell carcinoma, 1 carcinoid tumor, 1 pleomorphic carcinoma, 1 lymphoma, and 6 metastatic tumors. The 48 benign lesions (53%) had various diagnoses and were grouped based on their nature (A: 18 tuberculoma, 13 fungus; B: 5 focal organizing pneumonia (FOP), 5 abscesses; C: 7 benign tumors). Within benign tumors, there were 3 hamartomas, 3 sclerosing hemangiomas, and 1 inflammatory pseudotumor (Table 1).
Attenuations of SPN
The malignant SPN’s attenuations after contrast enhancement (EA) ranged from 28.6 to 98.0 HU and benign SPN’s EA from 18.3 to 86.8 HU. The mean attenuations of PreEA, EA, and NetEA of malignant SPN were 43.6±11.5, 67.9±14.0 and 26.3±13.4 HU, respectively. The mean attenuations of PreEA, EA, and NetEA of benign SPN were 26.3±13.4, 55.9±14.5, and 13.3±12.2 HU. The EA and NetEA of malignant SPN were significantly higher than those of the entire benign A–C groups (EA t=4.00, P<0.001; NetEA t=4.43, P<0.001) (Table 2). The cut-off EA of 61 HU or more gave 73.8% sensitivity, 62.5% specificity, 63% PPV, 77% NPV and 69% accuracy. The cut-off NetEA of 15 HU or more gave 83% sensitivity, 65%, specificity, 67% PPV, 82% NPV and 73% accuracy. Malignant SPN (mean 67.9 HU) had significantly higher enhancement than group A (mean 52.6 HU, P<0.001, 95% CI: 8.73, 21.81) and group B (mean 57.0 HU, P=0.025, 95% CI: −1.43, 20.34) whereas group C showed no significant difference (mean 68.1 HU, P=0.97). Net enhancements were higher in group B (mean 18.8 HU) than in group A (mean 8.8 HU) (P<0.001, 95% CI: 11.8, 23.18) (Table 3).

Full table

Full table
ROC curve
ROC curve was plotted for attenuations in PreEA, EA and NetEA of all samples. EA and NetEA showed meaningful curves for sensitivity and specificity (Figure 2).
Discussion
Thoracic radiologists routinely encounter SPN of various sizes, shapes and densities on CT scans. Making use of CT enhancement patterns of such indeterminate SPN could aid in diagnosis and patients would benefit from reduced cost and radiation dose caused by additional follow-up CT scans and invasive procedures like CT-guided biopsy or VATS.
Since Swensen et al. (2,3) emphasized that absence of significant lung nodule enhancement values 15 HU or less at CT is strongly predictive of benignity, various threshold attenuation values have been reported be useful for distinguishing malignant nodules from benign nodules at contrast-enhanced dynamic CT with single- or multi-detector row helical machines (4-7). This enhancement cutoff of 15 HU resulted in an excellent sensitivity of 98% but it only had 58% specificity. The general conclusion based on this report was that benign lesions usually enhance no more than 15 HU, whereas most of the malignant nodules develop more intensive enhancement, usually over 20 HU (7). On the other hand, Yi et al. reported that with 30 HU or more of net enhancement as a cutoff value in differentiation of malignant and benign nodules, sensitivity for malignant nodules was 99%, specificity was 54%, and accuracy was 78% (6). Malignant nodules showed significantly higher maximum relative enhancement ratio, shorter time to peak enhancement (6). Most malignant nodules had peak enhancement approximately two minutes after the administration of contrast media. In this study, the mean peak enhancement (MPE) of malignant nodules was reaching 98 HU, whereas relatively previous articles reported the MPE was approximately 40 HU (range, 41.9–46.5 HU) (8). In the more recent study, the evaluation of SPNs using dynamic contrast-enhanced MDCT was conducted by analyzing combined criteria for malignancy including wash in of contrast medium of 25 HU or greater and washout of 5–31 HU on 15 minutes-delayed imaging (4). The result of this study shows high sensitivity (94%), specificity (90%) and accuracy (92%) for detection of malignancy.
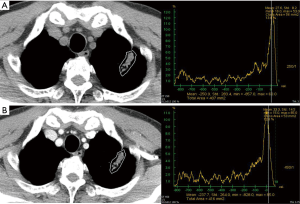
We used semi-automated ROI measurement method for more accurate attenuation measurement in order to differentiate malignant from benign SPN. In our study, EA and NetEA of malignant SPN were significantly higher than those of the benign group (enhancement t=4.00, P<0.001; net enhancement t=4.43, P<0.001), and the optimal cut-off NetEA of 15 HU or more yielded acceptable sensitivity (83%), specificity (65%) and accuracy (73%) for predicting malignant potential of SPN as compared with previous studies mentioned above. Our specificity was not low compared to the Swensen et al. and the accuracy was similar in comparison with the Yi et al. However, the reason for the subtle differences is probably that our study did not use dynamic CT. Second reason may be different measurement method itself between manually selected ROI and automatic total ROI. Third reason is likely to include too small nodules and heterogenous nodules such as cavitary tuberculoma (Figure 3), necrotic lung cancer (Figure 4), or lung abscess. This is a kind of preliminary reports of small populations.
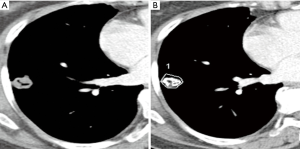
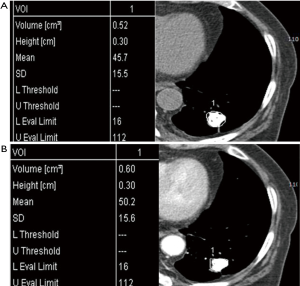
One study demonstrated solid non-calcified pulmonary nodules larger than 5 cm and their irregular, spiculated or lobulated margin increased the likelihood of nodule’s malignancy, whereas nodule density had no discriminative power; however, in contrast to our study, their density measurement of pulmonary nodules included air or air-bronchogram and hemorrhage within the tumor thus giving a wide range of densities (9). Our study used pre-determined range of densities from −100 to 200 HU in order to selectively measure only the solid enhancing portion of SPN because this range is a zone of soft tissue density area including cystic, necrotic, fatty portions, even hemorrhagic areas, but not calcifications, bony components, and airs. Indeed, our attempt is to eliminate the just calcific (almost benignity; about higher than 200 HU) or air densities (necrotic air cavity; about −1,000 HU) because of these materials are major errors of nodule homogeneity. There are also other advantages in segmentation of nodules by defining this range (from −100 to 200 HU). We can measure nodules in any size or shape even if we draw roughly outer periphery of nodule including the regional normal lung fields, because the surrounding lungs (about over −700 to 800 HU) are automatically eliminate (Figure 5). Sometimes, some pixels, which were just soft tissues, but not calcific or airs, were not covered as white figures on Lung Parenchyma Analysis (Siemens) (Figures 1,3). We are not sure the reason why. But we suppose that those areas are beyond 200 HU such as microcalcifications, highly vascularized areas or microvessel.
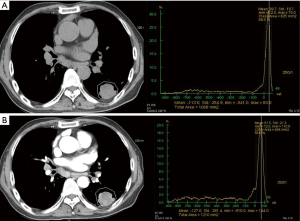
In addition to evaluating SPN’s malignant potential compared to all kinds of benign nodules, the present study described EA and NetEA of various benign SPN (tuberculoma, fungus, FOP, abscess and benign tumors) (Figures 6,7) independently and compared with malignant nodules. In our study, malignant SPN had significantly higher NetEA than that of group A (tuberculoma and fungus) and group C (benign tumor) while group B (FOP and abscess) showed no significant difference. Previous study by Zhang et al. demonstrated positive correlation between enhancement and vascularity within lung carcinoma, inflammatory pseudotumor, and tuberculoma in order of higher enhancement (10). Our findings in this study are consistent with the concept that malignant SPN expresses tumor angiogenesis and enhance stronger than the benign counterpart (11). As expected, the mean values of EA and NetEA of malignant SPN were significantly higher than those of benign SPN. In addition, the optimal cut-off NetEA value of 15 HU or more for distinguishing malignant from benign SPN, especially chronic granulomatous lesions, gave acceptable range of sensitivity, specificity and accuracy that are reasonably consistent with previous studies. There was no significant NetEA difference between malignant and benign group B (FOP, abscess) SPN. No significant difference in EA was found between malignant SPN and group B and C. Like this, the results of our study between malignant and FOP and abscess was relatively corresponded to those of previous study (4). They reported that malignant and some non-specific benign nodules showed persistent enhancement without washout. In fact, we experienced in clinical fields that many FOP, abscess wall and some benign tumor such as sclerosing hemangioma are nearly similar to malignant tumor in point of view of enhancement pattern. Hittmair and Zhang found similar results in that benign pulmonary nodules of inflammatory nature displayed strongest enhancement (12,13). Upon encountering such SPN, considering other parameters of malignancy such as large size, spiculated or lobulated margin and interval growth could help in correct diagnosis.
CT assessment was most effective for lesions 2.0 cm or less in diameter (14). Small solid-density pulmonary adenocarcinoma has poor prognosis even if they are less than 2.0 cm in size (15). Other studies limited selection of SPNs to 5 mm to 3 cm in diameter with homogeneous density of spherical shape without cavitation, necrosis, calcifications and fat because this criterion allows for optimal manual measurement of central ROI (2,7). Even for larger SPN, internal cavitation and air-bronchogram are CT features frequently associated with malignancy and manual selection of solid portion in mixed densities may not be an easy task (16,17). Using semi-automated ROI measurement, our study was able to include SPN of any sizes from small to large and of various internal contents via comprehensive inclusion of solid portion of SPN under a preset range of densities. The mean values of our results in both malignant and benign nodules tend to be lower than those of previous reports. The reason for this phenomenon was that our semi-automated method included all heterogeneous necrotic portions of solid tumors, whereas previous manual methods excluded non-solid low-density areas involuntarily.
There are several limitations that we need to address. First, the study’s retrospective nature is prone to selection bias. The number of total samples was relatively small (n=90) which is subject to lower statistical power. With respect to technical aspects, the two CT scanners used different software but we assumed their algorithm and ability to measure attenuation were probably the same. All contrast-enhanced CT images were acquired 70 seconds after contrast injection which might have led to variations related to patients’ breath holding. However, we assumed contrast media enter the bronchial arteries in 11–19 seconds and more than half of contrast would have reached extravascular space of most tissues in 60 seconds (18). However, we wanted all CT data to be as similar to clinical settings as possible and for that reason, dynamic contrast-enhanced CT scan wasn’t applied for this study. Finally, all CT scans were sliced at 3-mm thickness, which could have neglected ground-glass elements.
Conclusions
In conclusion, our study demonstrated a novel approach in measuring attenuation of SPN of various sizes, shapes and internal contents. Compared with similar previous studies on SPN density measurement, we showed similar sensitivities and specificities in predicting malignancy thereby proving the applicability of semi-automated ROI measurement on CT in evaluating SPN to a larger extent.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional review board of our institution (Bucheon St. Marys Hospital) (No. HC16RASI0101) and written informed consent was obtained from all patients.
References
- Gómez-Sáez N, Hernández-Aguado I, Vilar J, et al. Lung cancer risk and cancer-specific mortality in subjects undergoing routine imaging test when stratified with and without identified lung nodule on imaging study. Eur Radiol 2015;25:3518-27. [Crossref] [PubMed]
- Swensen SJ, Viggiano RW, Midthun DE, et al. Lung nodule enhancement at CT: multicenter study. Radiology 2000;214:73-80. [Crossref] [PubMed]
- Swensen SJ, Brown LR, Colby TV, et al. Lung nodule enhancement at CT: prospective findings. Radiology 1996;201:447-55. [Crossref] [PubMed]
- Jeong YJ, Lee KS, Jeong SY, et al. Solitary pulmonary nodule: characterization with combined wash-in and washout features at dynamic multi-detector row CT. Radiology 2005;237:675-83. [Crossref] [PubMed]
- Schaefer JF, Vollmar J, Schick F, et al. Solitary pulmonary nodules: dynamic contrast-enhanced MR imaging--perfusion differences in malignant and benign lesions. Radiology 2004;232:544-53. [Crossref] [PubMed]
- Yi CA, Lee KS, Kim EA, et al. Solitary pulmonary nodules: dynamic enhanced multi-detector row CT study and comparison with vascular endothelial growth factor and microvessel density. Radiology 2004;233:191-9. [Crossref] [PubMed]
- Choromańska A, Macura KJ. Evaluation of solitary pulmonary nodule detected during computed tomography examination. Pol J Radiol 2012;77:22-34. [Crossref] [PubMed]
- Yamashita K, Matsunobe S, Tsuda T, et al. Solitary pulmonary nodule: preliminary study of evaluation with incremental dynamic CT. Radiology 1995;194:399-405. [Crossref] [PubMed]
- Xu DM, van Klaveren RJ, de Bock GH, et al. Limited value of shape, margin and CT density in the discrimination between benign and malignant screen detected solid pulmonary nodules of the NELSON trial. Eur J Radiol 2008;68:347-52. [Crossref] [PubMed]
- Zhang Z, Zhang C, Wu P, et al. Comparison of enhanced thin CT sections with pathologic findings in pulmonary carcinoma, inflammatory, pseudo-tumor and pulmonary tuberculoma. Zhonghua Zhong Liu Za Zhi 2002;24:173-7. [PubMed]
- Tateishi U, Nishihara H, Watanabe S, et al. Tumor angiogenesis and dynamic CT in lung adenocarcinoma: radiologic-pathologic correlation. J Comput Assist Tomogr 2001;25:23-7. [Crossref] [PubMed]
- Hittmair K, Eckersberger F, Klepetko W, et al. Evaluation of solitary pulmonary nodules with dynamic contrast-enhanced MR imaging--a promising technique. Magn Reson Imaging 1995;13:923-33. [Crossref] [PubMed]
- Zhang M, Kono M. Solitary pulmonary nodules: evaluation of blood flow patterns with dynamic CT. Radiology 1997;205:471-8. [Crossref] [PubMed]
- Siegelman SS, Khouri NF, Leo FP, et al. Solitary pulmonary nodules: CT assessment. Radiology 1986;160:307-12. [Crossref] [PubMed]
- Ikehara M, Saito H, Kondo T, et al. Comparison of thin-section CT and pathological findings in small solid-density type pulmonary adenocarcinoma: prognostic factors from CT findings. Eur J Radiol 2012;81:189-94. [Crossref] [PubMed]
- Gadkowski LB, Stout JE. Cavitary pulmonary disease. Clin Microbiol Rev 2008;21:305-33. [Crossref] [PubMed]
- Onn A, Choe DH, Herbst RS, et al. Tumor cavitation in stage I non-small cell lung cancer: epidermal growth factor receptor expression and prediction of poor outcome. Radiology 2005;237:342-7. [Crossref] [PubMed]
- Littleton JT, Durizch ML, Moeller G, et al. Pulmonary masses: contrast enhancement. Radiology 1990;177:861-71. [Crossref] [PubMed]


