
Favorable clinical application for segmental bronchial closure based on experiment results
Introduction
Stapling instruments are indispensable to ensure the safety of thoracoscopic segmentectomy (TS) and to reduce the surgical duration, intraoperative blood loss, and the risk of prolonged air leakage after surgery. Thoracic surgeons frequently use stapling instruments to close the bronchi in TS and thoracotomy. Of the complications associated with the use of a stapling instrument, bronchopleural fistula (BPF) formation is considered among the most severe because of the deterioration of quality of life and increased risk of death. It is estimated that the incidence of BPF formation after thoracoscopy lobectomy is 0% to 6.3% in various pulmonary resections (1-7).
A meta-analysis and a multi-institutional review comparing the stapling instrument and sutures toward the bronchial closure have emphasized a lack of evidence of manual sutures superiority (8,9). Thoracic surgeons should be aware of the thickness of the bronchus when choosing between the use of a stapler vs. manual closure because closure of the bronchus is dependent on the hardness and bronchial outer diameter (BOD), which are important factors for segmentectomy of the segmental or subsegmental bronchus (SSB) because the BOD is relatively smaller and widely varied, as compared with those in lobectomy. Several closure methods, such as ligation, the use of a powered linear cutter (PLC) (ethicon®, USA), and clipping, have been described, but there is limited information regarding complications associated with BPF formation after TS. We previously summarized the benefits of a powered vascular staple (PVS) (PLC, ethicon®, USA) for closure of the SSB, in accordance with current guidelines and based on our experience, and found that SSB transection was easier and smoother with the use of PVS than with a PLC because of the configuration of a PVS (10). Because PVS can be officially available for the subsegmental or segmental bronchi which surgeon can compress by thickness of 1 mm in Japan (10).
Information regarding the safety of stapling instruments for SSB is limited and little is known about the frequency of BPF formation. Therefore, it is necessary to verify the safety profile of a PVS for SSB closure in experimental and clinical studies. In the first part of this study, the threshold of air leak pressure after SSB closure with a PVS was measured in a cadaver pig model, whereas the aim of the second part of this study was to investigate the usefulness of SSB closure in 217 TS procedures and the morbidity of BPF formation at 6 months or more after surgery.
Methods
Experimental design
Cadaveric pig chest organs, including the esophagus, lungs, and heart, were purchased from a commercial supplier and refrigerated. The trachea, bronchus, and lungs were dissected. Then, the pressure experiment was conducted using 1 to 2 segmental bronchi per body. A total of 30 bronchi were categorized into the following three groups: small (group S; 4–8 mm at BOD; n=8), medium (group M; 9–10 mm; n=9), and large (group L; >10 mm; n=13). We discovered that the distal edge of the stapled line caused leak in large (Figure 1A). Therefore, we sutured the weakest site along the stapled line using a single 4-0 absorbable monofilament (4AMF) (Figure 1B). Organs that were resutured were classified into the reinforced group (group R; Figure 1B). Once the SSB was transected with a PVS or PLC, the opposite main bronchus was closed with clamp forceps. Afterward, the pressure was slowly increased using an intubation tube with an internal diameter of 7.5 mm, and air leakage through the stapled stump was observed in a water tank. Maximum pressure was defined as up to 400 cmH2O.

Study design
The study protocol was approved by the Ethics Committee of Aichi Cancer Center and conducted in accordance with the guidelines of the local Institutional Review Board (permit No. 2017–1–296). The PVS closures of SSBs during various TS were previously presented (10). Between January 2013 and September 2017, a total of 217 patients underwent TS at Aichi Cancer Center Hospital. All surgeries were performed by the same two experienced surgeons and a dedicated TS team. All patients were followed-up for more than 6 months as outpatients. Preoperatively, three-dimensional reconstructions were produced using thin-sliced computed tomography (CT) with a commercial workstation (Synapse Vincent, Fuji Film Co., Ltd., Tokyo, Japan). The resection-planed SSB was visualized, and the BOD was measured (Figure 1C). For TS, the boundaries of the lung segments were usually identified by the intravenous indocyanine green injection technique after transection of the subsegmental and SSB, vessels (arteries and veins), and bronchial arteries (11,12). The SSB was transected with a PLC or PVS, or ligated based on BOD according to circumstances. The segmental boundary was usually made with a PLC. In this study, the maximum BOD was adopted, and the multiple SSB were transected in TS. Every patient underwent thin-sliced CT before surgery and at 6 and 12 months afterward. Chest X-rays were obtained before surgery and 3 months afterward. All postoperative complications were recorded.
Statistical analysis
The data were analyzed with SPSS software (version 17.0J; SPSS, Inc., Chicago, IL, USA). The Mann-Whitney U test was used for comparisons between two groups, whereas the Kruskal-Wallis test was used for comparisons of more than two groups. The correlation for 2 markers was analyzed by the Pearson’s correlation coefficient. A probability (P) value of <0.05 was considered statistically significant.
Results
Threshold of air leak pressure and closure of segmental bronchi using a PVS
The mean BOD in groups S, M, L, and R was 6.4±1.3, 9.4±0.5, 12.1±1.3, and 11.5±1.2 mm, respectively. There was no significant difference in BOD between groups L and R (P=0.36).
The mean compressed thickness was greatest in group L (1.6±0.3 mm), followed by groups M and S (1.3±0.2 and 1.2±0.3 mm, respectively). There were significant differences between groups M and L and between groups S and L (P=0.02 and P<0.01, respectively), but not between groups S and M (P=0.34). There was a strong correlation between BOD and the compressed thickness (P<0.01, R=0.63) (Figure 2A).

The mean air leak pressure was the highest in group M (314.7±85.8 cmH2O), followed by groups S and L (301.4±85.3 and 185.5±82.8 cmH2O, respectively). There was a significant difference in mean air leak pressure between groups S and L, and between groups M and L (P=0.02 and P<0.01, respectively), but not between groups S and M (P=0.85) (Figure 2B). While the resistance to pressure of the segmental stump closed by manual placement of sutures (n=3) and with the use of a PLC (n=3) could tolerate up to 400 cmH2O.
Subsequently, we evaluated the efficacy of reinforcement of the staple line with 4AMF. The mean pressure of air leakage in group R (n=6; 303.0±71.0 cmH2O) was equivalent to that in BOD of the SSB with equal or less than in 10 mm (n=17; 308.5±83.2 cmH2O) (P=0.94) (Figure 2B).
The incidence of BPF formation after TS with a PLC, PVS, or ligation for SSB closure
During the study period, 217 patients underwent TS for non-small-cell lung carcinoma (n=161, 74.2%), metastasized disease (n=50, 23.0%), and benign disease (n=6, 2.8%). As summarized in Table 1, SSB was transected with a PLC in 127 (58.5%) patients, a PVS in 73 (33.6%), and by ligation in 17 (7.8%). The mean follow-up duration was 27.2±13.7 (range, 10.2–64) months and 94.9% (206/217) of patients were still alive at the conclusion of the study.
Full table
The mean preoperative transection-planned BOD of the SSB was greatest with a PLC (7.3±1.6 mm, P<0.01), followed by a PVS and ligation (6.2±1.4 and 5.2±1.4 mm, respectively, P=0.69) (Figure 2C). The BPF formation did not occur with any SBB closure technique.
Discussion
BPF formation is a life-threatening condition but rare in segmentectomy. In addition, lymph node dissection with disconnection of the bronchial arteries can cause ischemic bronchitis and increase the risks of mortality and morbidity. Recent several studies published within 5 years have reported a relationship between the incidence of BPF formation after pulmonary resection and pneumonectomy (range, 2.9% to 6.3%), as well as bilobectomy (range, 2.2% to 2.5%), and lobectomy (range, 0.5% to 3.0%) followed by segmentectomy (range, 0% to 0.3%) in thoracic surgery (1-7,13). To the best of our knowledge, only one study conducted in Japan referred to the incidence of BPF after TS. Oizumi et al. reported their experience with 208 patients who underwent segmentectomy, which included 41 cases of open thoracotomy and 167 of thoracoscopy, and observed only one case of BPF formation (0.3%) among 297 bronchial closures, which occurred at 6 months after surgery during upper division segmentectomy of the left upper lobe using a stapler (13). The various causes of BPF formation are reportedly involvement of the right side, closure of the bronchial stump, extensive lymphadenectomy, smoking status, duration of mechanical ventilation after pneumonectomy, lower bilobectomy, male, low body mass index, and revision surgery after bilobectomy (1-6). In the present study, there was no complication of BPF formation. In the present study and that conducted by Oizumi et al., complications of BPF formation occurred after TS. However, further investigations are needed to determine the actual incidence of this complication. A possible reason for BPF formation is a decrease in devascularization and preservation of the blood supply in the SSB stump from the remnant surrounding the lung parenchyma, as compared with lobectomy or pneumonectomy (14,15). Another possible cause is that the proportion of complete or extensive mediastinal lymph node dissection is relatively lower with segmentectomy than with lobectomy or pneumonectomy. Several recent retrospective studies reported that the prognosis of segmentectomy with radical mediastinal lymph node dissection is equivalent to that of lobectomy for patients with cT1N0M0 non-small cell lung cancers despite the existence of worldwide standards for treatment of invasive lung cancers (16). The indication for segmentectomy is radiological heterogeneous ground grass opacity (a solid component detected only in the lung) or up to at most 2 mm in mediastinal setting within a diameter of 20 mm in maximum diameter on CT based on our previous data (17-19) and some representative studies (20).
We previously summarized the intraoperative usage of a PVS for small SSB during TS (10). Although the number of cases was greater in the present study, no BPF formation was identified. Oizumi et al. concluded that ligation is safe for closure of bronchial stumps with small diameters (13). In this study, the mean BOD of bronchi resected by PVS was 6.2±1.4 mm, which was larger than with ligation (5.2±1.4 mm), however; significantly smaller than with a PLC (7.3±1.6 mm) on preoperative CT. The mean BOD in this study was close to that in previous reports, and the goal of certain transection of SSB was to achieve a safe and feasible transection up to 1 mm in thickness during compression. Several authors reported that the maximum endobronchial pressure may be as high as 200 mmHg under clinically urgent circumstances, including cough and sneeze, and insufficient stump closure could lead to stump failure (21,22). Although it was difficult to compress SSB up to 1 mm or less, the PVS closure of SSB for BOD of <10 mm could withstand the urgent maximum pressure. In addition, reinforcement by manual placement of additional sutures with 4AMF revealed significantly higher resistance to intraluminal inflation pressure as compared with closure using a PVS and may contribute better early postoperative tolerance to abnormal pressure before sound healing of the SSB stump is achieved. Further studies are warranted to determine how long BOD can be tolerated by either a PLC or PVS.
The clinical use of a PVS for SSB closure should be altered accordingly. However, two questions remain unanswered: (I) how much the intraluminal inflated pressure appears in postoperative days; and (II) what is the ideal compressed thickness of the segmental bronchi. In this study, treatment efficacy was assessed using a pig model by auto-suturing with a PVS. Although little is known about the integrity of SBB closure using a PVS and the natural threshold of tolerability during the early postoperative period, common techniques for SSB closure can be adapted with manual suture placement or with a PLC, as simple ligation according to the segmental bronchial size is of paramount importance because of the rare morbidity of BPF formation. Certainly, in this experimental setting, the resistance to pressure of the segmental stump closed by manual placement of sutures (n=3) and with the use of a PLC (n=3) could tolerate up to 400 cmH2O. In contrast, the results obtained in this study showed that the use of a PVS was less resistant to pressure and subsequent air leakage, as compared with the use of a PLC. This difference can be explained by referring to the double (PVS) or triple (PLC) rows of staple lines. Therefore, closure with a double staple line should provide sufficient resistance against those clinical urgent circumstances if thoracic surgeons can compress the SSB up to approximately 1 mm. If compression is not possible in the cases of PVS use with a larger BOD or equal or greater than two segments, reinforcement of SSB with 4AMF should be considered.
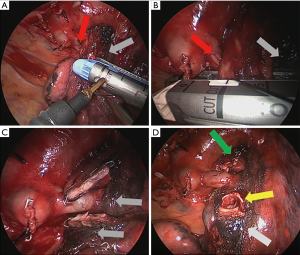
While we repeated the experiment, the sites along the PVS stapled line were insufficient, especially with a larger BOD (>10 mm), as progressively increased pressure caused a puncture. Therefore, a single suture using 4AMF was insufficient to reinforce the SSB stump. However, a previous study demonstrated that hand suturing of the bronchi tolerated higher inflation pressure as compared with the use of a stapler (23). In addition, simple closure with two suture lines could tolerate up to 400 cmH2O (n=3, data not shown). The two main structural features of PVS are the shortened width of the anvil and an additional 10° of total articulation, which could be applied with relatively narrow cases, such as with lymph node calcification and increased thickness and adhesion of the surrounding tissue due to multifocal inflammation under the segmental bronchi, especially in TS. As shown in Figure 3A,B,C,D, we inevitably used a PVS instead of a PLC for the left upper bronchial segment because of the narrow space due to lobar lymph node calcification and to avoid excessive tension of the superior lingular segmental artery. Therefore, subsequent reinforcement with an additional stapled line with a 4AMF was performed. During the ligation, the staples for the resected stump were completely undone. Fortunately, no BPF formation was observed during a follow-up interval of more than 1 year. We recommend the use of a PVS for segmental bronchi that are compressed by less than 10 mm to improve the ability of the thoracic surgeons to quickly manage such a difficult situation.
The major limitations of this study included the retrospective and single institutional nature of the clinical data. To provide meaningful data on definitive regulation and to avoid errors, a prospective, multicenter study is required, which is in the planning phase. The clinical application according to standard regulations demonstrated by this study can only be defined by exploiting the relevant and reliable clinical outcomes based on the experimental design.
In conclusion, based on our experimental results and clinical experience, the proper selection of a PVS for segmental bronchus should contribute to the safety, feasibility, and success of the procedure. Although further prospective studies will be required, reinforcement with a stapled line with the use of a PVS should be adequate to prevent deterioration due to breakdown pressure.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ethics Committee of Aichi Cancer Center and conducted in accordance with the guidelines of the local Institutional Review Board (permit No. 2017–1–296).
References
- Pforr A, Pagès PB, Baste JM, et al. A Predictive Score for Bronchopleural Fistula Established Using the French Database Epithor. Ann Thorac Surg 2016;101:287-93. [Crossref] [PubMed]
- Hu XF, Duan L, Jiang GN, Wang H, et al. A clinical risk model for the evaluation of bronchopleural fistula in non-small cell lung cancer after pneumonectomy. Ann Thorac Surg 2013;96:419-24. [Crossref] [PubMed]
- Di Maio M, Perrone F, Deschamps C, et al. A meta-analysis of the impact of bronchial stump coverage on the risk of bronchopleural fistula after pneumonectomy. Eur J Cardiothorac Surg 2015;48:196-200. [Crossref] [PubMed]
- Thomas PA, Falcoz PE, Bernard A, et al. Bilobectomy for lung cancer: contemporary national early morbidity and mortality outcomes. Eur J Cardiothorac Surg 2016;49:e38-43; discussion e43.
- Thomas PA, Berbis J, Baste JM, et al. Pneumonectomy for lung cancer: contemporary national early morbidity and mortality outcomes. J Thorac Cardiovasc Surg 2015;149:73-82. [Crossref] [PubMed]
- Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg 2012;256:487-93. [Crossref] [PubMed]
- Okada M, Tsutani Y, Ikeda T, et al. Radical hybrid video-assisted thoracic segmentectomy: long-term results of minimally invasive anatomical sublobar resection for treating lung cancer. Interact Cardiovasc Thorac Surg 2012;14:5-11. [Crossref] [PubMed]
- Zakkar M, Kanagasabay R, Hunt I. No evidence that manual closure of the bronchial stump has a lower failure rate than mechanical stapler closure following anatomical lung resection. Interact Cardiovasc Thorac Surg 2014;18:488-93. [Crossref] [PubMed]
- Yano M, Yokoi K, Numanami H, et al. Complications of bronchial stapling in thoracic surgery. World J Surg 2014;38:341-6. [Crossref] [PubMed]
- Kuroda H, Yoshida T, Sakao Y. A powered vascular staple for the application of segmental bronchial closure in thoracoscopic anatomic segmentectomy. J Thorac Dis 2017;9:5352-4. [Crossref] [PubMed]
- Kuroda H, Yoshida Y, Arimura T, et al. Novel development of Spectra-A using indocyanine green for segmental boundary visibility in thoracoscopic segmentectomy. J Surg Res 2018;227:228-33. [Crossref] [PubMed]
- Iizuka S, Kuroda H, Yoshimura K, et al. Predictors of indocyanine green visualization during fluorescence imaging for segmental plane formation in thoracoscopic anatomical segmentectomy. J Thorac Dis 2016;8:985-91. [Crossref] [PubMed]
- Oizumi H, Kato H, Endoh M, et al. Management of Bronchial Stumps in Anatomic Lung Segmentectomy. Ann Thorac Surg 2016;101:2120-4. [Crossref] [PubMed]
- Nomori H, Mori T, Ikeda K, et al. Segmentectomy for selected cT1N0M0 non-small cell lung cancer: a prospective study at a single institute. J Thorac Cardiovasc Surg 2012;144:87-93. [Crossref] [PubMed]
- Ishihara T, Nemoto E, Kikuchi K, et al. J Does pleural bronchial wrapping improve wound healing in right sleeve lobectomy? J Thorac Cardiovasc Surg 1985;89:665-72. [PubMed]
- Kato R, Onuki AS, Watanabe M, et al. Tracheal reconstruction by esophageal interposition: an experimental study. Ann Thorac Surg 1990;49:951-4. [Crossref] [PubMed]
- Sakao Y, Kuroda H, Mun M, et al. Prognostic significance of tumor size of small lung adenocarcinomas evaluated with mediastinal window settings on computed tomography. PLoS One 2014;9:e110305. [Crossref] [PubMed]
- Sakakura N, Inaba Y, Yatabe Y, et al. Estimation of the pathological invasive size of pulmonary adenocarcinoma using high-resolution computed tomography of the chest: A consideration based on lung and mediastinal window settings. Lung Cancer 2016;95:51-6. [Crossref] [PubMed]
- Kuroda H, Mori S, Tanaka H, et al. Prognostic significance of combined radiologic imaging modalities for prognosis of clinical IA adenocarcinomas. Oncotarget 2017;9:10745-53. [PubMed]
- Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol 2011;6:751-6. [Crossref] [PubMed]
- Ansell TK, McFawn PK, Mitchell HW, et al. Bronchodilatory response to deep inspiration in bronchial segments: the effects of stress vs. strain. J Appl Physiol 1985;2013:505-13. [PubMed]
- Ludwig C, Hoffarth U, Haberstroh J, et al. Resistance to pressure of the stump after mechanical stapling or manual suture. An experimental study on sheep main bronchus. Eur J Cardiothorac Surg 2005;27:693-6. [Crossref] [PubMed]
- El-Gamel A, Tsang GM, Watson DC. The threshold for air leak: stapled versus sutured human bronchi, and experimental study. Eur J Cardiothorac Surg 1999;15:7-10. [Crossref] [PubMed]


