
Particulate matter disrupts airway epithelial barrier via oxidative stress to promote Pseudomonas aeruginosa infection
Introduction
Particulate matter (PM), as carrier and catalyst of many harmful substances, can enter the bronchi and alveoli through inspiration, and even into the blood circulatory system, which has deleterious effects on multiple organ systems (1,2). At present, there are many studies focused on the relationships between PM and lung infection. Sigaud et al. (3) showed that PM2.5 particles could reduce the function of neutrophils and macrophages in mice and increase the risk of infection with streptococcus pneumoniae. Zhao et al. (4) found that the number and activity of NK cells and alveolar macrophages significantly decreased in mice after the inhalation of PM2.5 particles, which increased the susceptibility to staphylococcus aureus in the lungs. Some researches demonstrated that exposing alveolar macrophages to cigarette smoke, cooking fumes and other particles could cause a significant impairment in their number and function, which resulted in a decline in the scavenging ability of bacteria, aggravated lung infection and inflammation, and increased the chances of infection of various opportunistic pathogens (5,6).
Pseudomonas aeruginosa (P. aeruginosa), a kind of gram negative bacillus, is characterized by secondary infection which occurs in patients with low body resistance, such as cystic fibrosis and chronic obstructive pulmonary disease (7). Psoter et al. (8) reported that an increase in PM2.5 exposure concentration of 10 µg/m3 was associated with a 24% increased risk of P. aeruginosa infection, which indicates that PM may contribute to P. aeruginosa infection in the respiratory system.
Tight junctions (TJs) are the important protein complexes at cell-cell interfaces that connect adjacent cells with each other to form lung epithelial barrier (9). Claudins are the main proteins constitute the structure of TJs. According to whether they can form paracellular channels or whether they control paracellular permeability, claudins have been categorized as pore forming claudins or sealing claudins (10,11). Lung epithelium tends to favor expressions of sealing claudins including claudin-1 and -5 (12). Occludin, a key regulator of TJs stability and function, is closely related to the transcription of claudin-1 (13). It was reported that PM increased MUC5AC expression by downregulating claudin-1 in human airway cells (14). In addition, studies have demonstrated that P. aeruginosa caused transient disruption of TJs and downregulated the claudin-1, claudin-4, and occludin (15). Previously, our group has found that claudins released into the alveolar compartment was highly related to barrier function loss after P. aeruginosa installation (16). However, whether PM would promote the invasion of P. aeruginosa by disruption of TJs is still unknown.
We hypothesize that PM promotes ROS generation, leading to a disruption of TJs proteins and the invasion of P. aeruginosa. To test this, we investigate the effect of PM on human bronchial epithelial cells and mice upon P. aeruginosa infection.
Methods
Cell culture and PM treatment
The human bronchial epithelial cell lines (BEAS-2B) were purchased from Shanghai Institute of Cell Biology, Chinese Academy of Sciences, and were cultured in high glucose dulbecco’s modified eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS), 100 IU/mL penicillin and 100 µg/mL streptomycin at 37 °C in a humidified atmosphere with 5% CO2. PM Standard Reference Material (SRM) 1649b was obtained from the National Institute of Standards and Technology (NIST, Gaithersburg, Maryland, USA). It is composed of selected polycyclic aromatic hydrocarbons (PAHs), nitro-substituted PAHs (nitro-PAHs), polychlorinated biphenyl (PCB) congeners, chlorinated pesticides, and inorganic constituents. And it is dispersed in all kinds of sizes. Stock solutions of PM (4 mg/mL) were prepared by phosphate buffered saline (PBS) and were stored at −20 °C. Cells were seeded in 10 cm2 dishes, 6- or 96-well plates, and exposed to PM at a final concentration of 50, 200 and 400 µg/mL, respectively.
P. aeruginosa preparation
P. aeruginosa strain employed was PAO1 with green fluorescent protein (GFP, excitation 488 nm, emission 507 nm, kindly provided by University of California, San Francisco, USA). In each group of experiments, PAO1-GAP was streaked onto a pseudomonas cetrimide agar (PCA, Thermo Fisher, Hampshire, England) medium plate and cultured for 18 to 24 h at 37 °C. Then, a single colony was inoculated in PCA medium by rotary shaking at 37 °C for 16 to 18 hours until the bacteria reached log-phase. Subsequently, 1 mL bacterial suspension was washed thrice with PBS and suspended by PBS.
Cell viability assay and LDH release assay
Cell viability was assessed using Cell Counting Kit-8 (CCK-8, Beyotime, Shanghai, China). BEAS-2B cells were seeded into 96-well plates overnight and then treated with PM at doses of 0, 50, 200 and 400 µg/mL for 2, 6, 12, 24 and 48 hours, respectively. After treatment, CCK-8 solution was added to each well at a ratio of 1:10 for another 1 hour at 37 °C. The absorbance at 450 nm was read by a microplate reader (FlexStation® 3; Molecular Devices, San Jose, USA). The lactate dehydrogenase (LDH, MAK066, Sigma-Aldrich, St. Louis, USA) release assay was performed according to the manufacturer’s instructions.
Reactive oxygen species (ROS) assay
The intracellular ROS was detected by 2’,7’-Dichlorodihydrofluorescein diacetate (DCFH-DA, Beyotime, Shanghai, China). BEAS-2B cells were plated in 6-well plates at a density of 2.5×105 cells per well and cultured for 24 hours. After treated with PM for 24 hours, cells were incubated with DCFH-DA at the final concentration of 10 µmol/L for 20 min at 37 °C in the dark. Then, the fluorescence of 2,7-dichlorofluorescein (DCF) was monitored using flow cytometer (BD FACScantoTM, San Jose, California, USA). We used the mean fluorescence intensities (MFI) to compare the fluorescence intensity.
Immunofluorescence assay
A sterilized coverslip was laid into each well of a 6-well plate and cells were seeded onto the coverslip at a density of 2.5×105 cells/mL. BEAS-2B cells were pretreated with N-acetylcysteine (NAC, 5 mM) 1 hour before 24 hours PM (200 µg/mL) exposure, then cells were infected by PAO1 (at a multiplicity of infection of 10, MOI 10). Cells were fixed with 4% formaldehyde before being blocked by 5% bovine serum albumin (BSA). After incubation with anti-zonula occluden (ZO)-1, anti-occludin, or anti-claudin-1 antibody [Cell Signaling Technology (CST), Boston, Massachusetts, USA] at 4 °C overnight, cells were incubated with fluorescein isothiocyanate (FITC, excitation 494 nm, emission 518 nm, CST) or cyanine dye (cy3, excitation 555 nm, emission 564 nm, CST)-conjugated secondary antibodies for 2 hours at room temperature. Subsequently, the cells were treated with 4’, 6-diamidino-2-phenylindole (DAPI, excitation 345 nm, emission 455 nm, Sigma-Aldrich, St. Louis, USA) 5 min. The results were observed and photographed by fluorescence microscopy.
Invasion assay assessed by colony forming units (CFU) assay and confocal microscopy
The two experiments were performed as described previously with modifications in this study (17,18). When grown to approximately 50–60% confluency, cells were cultured with PM for 24 hours and subsequently infected with PAO1 at a MOI of 10 by replacing the medium with DMEM containing 50 µL bacteria suspension (OD600 0.25≈1×108CFU/mL) for 2 hours. After vigorously washed thrice with PBS, cells were added by 200 mg/mL gentamicin sulfate (Sigma-Aldrich, St. Louis, USA) for 3 hours. Then, cells were lysed with 0.1% Triton X-100 (Sigma-Aldrich, St. Louis, USA). The lysates were serially diluted and plated onto PCA plates in triplicate. The CFUs represented the numbers of intracellular bacteria (19). In confocal microscopy, cells were seeded on glass coverslips for 24 hours. Subsequently, cells were cultured with PM for 24 hours, infected with PAO1 at a MOI of 10 for 2 hours, and bacteria located outside the cells were killed using 200 mg/mL gentamicin for 3 hours. Next, 4% paraformaldehyde was added to wells to fixed cells. Then DAPI was added to stain the nuclei. The cell membranes were stained with 1, 19-dioctadecyl-3, 3, 39, 39-tetramethylindocarbocyanine perchlorate (DiI, excitation 549 nm, emission 565 nm, Beyotime, Shanghai, China) for 10 min before the coverslips were removed and tested by laser confocal microscope (Leica TCS SP5 II, Leica Microsystems, Wetzlar, Germany). The intracellular GFP-PAO1 was counted and expressed as intracellular PAO1 per cell.
Trans-epithelial electrical resistance (TEER)
The TEER was measured with the EVOM2 system (Epithelial Voltohmmeter, America). Briefly, BEAS-2B cells were seed on the upper chamber of the transwells. The mean of three measurements per insert was determined. The electrical resistance of insert membranes without cells was subtracted, and the real resistance values were multiplied with the total surface area of the inserts (0.33 cm2) as described before (20).
Animals
Twenty-four C57BL/6J mice (12 male; 12 female) were purchased from SLAC Laboratory Animals (Shanghai Laboratory Animal Center, Shanghai, China), 8–10 weeks old, and certified as specific pathogen-free. They were housed for one week (temperature 20–24 °C, humidity 40–60%, lights on 8 a.m.–8 p.m.), then averagely and randomly divided into four groups (Six mice per group). Each group was inoculated by instilling PBS or PM (0.5, 2, or 4 mg/kg) respectively three times for 72 hours (21) before P. aeruginosa (1×106 CFU, 24 hours) infection as previously described (22). The studies were approved by the Animal Care and Use Committee of Fudan University (certificate number: 2016-026), Shanghai.
Lung histology
Following euthanasia with avertin, the lungs were isolated and immersed in 10% formalin, dehydrated in ethanol and embedded in paraffin. Then the hematoxylin and eosin staining was performed and lung injury was evaluated as described previously (19).
Lung bacterial burden
At 24 hours after infection, mice were euthanized and lungs were removed in a sterile fashion. The lungs were weighted and homogenized in 1 mL sterile PBS. Five hundred microlitres of the blood were collected aseptically. Then homogenized lung and blood samples were serially diluted and plated onto PCA plates in triplicate, which were incubated at 37 °C overnight prior to bacterial enumeration. The CFUs represented the number of lung and blood bacteria (19).
Western blot assay
BEAS-2B cells and lung tissues of mice from each group was obtained and electrophoresed, transferred to the Poly vinylidene fluoride (PVDF) membranes, and then incubated with anti-occludin antibody, anti-claudin-1 antibody, and anti-GADPH antibody (CST, Boston, MA, USA) overnight at 4 °C. After washing thrice in TBST, membranes were incubated with a peroxidase-conjugated secondary antibody (1:1,000 dilution, CST) for 1 hour at room temperature and detected by enhanced chemiluminescence (Thermo Fisher, Hampshire, England).
Statistical analysis
Each in vitro experiment was repeated for three times with three duplication in each group. All data were expressed as mean ± standard deviation. Comparisons among multiple groups were performed with one-way analysis of variance (ANOVA) and Bonferroni adjusted pairwise comparisons were used for post-hoc pair test. Spearman correlation was used for correlation analysis. All analyses were two-sided and performed with GraphPad Prism 5 (GraphPad Software, Inc., San Diego, California, USA). P<0.05 was considered statistically significant.
Results
Toxicity effects of PM on the human bronchial epithelial cells
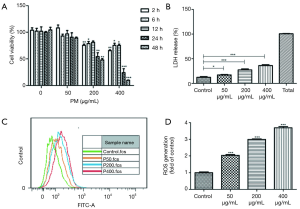
BEAS-2B cells were exposed to 0, 50, 200 and 400 µg/mL PM for 2, 6, 12, 24 and 48 hours, respectively (Figure 1A). Although no significant changes of intracellular formazan production in BEAS-2B cells were observed, there was a visible decrease trend after exposure to 50 µg/mL of PM for 2–48 hours. When BEAS-2B cells were exposed to 200 or 400 µg/mL of PM for 2–48 hours, we observed a significant decrease of cell viability except for the group being exposed to 200 μg/mL of PM for 12 hours. In addition, the results demonstrated that exposing to PM of 200 µg/mL for 24 hours reduced BEAS-2B viability to almost 50%. Therefore, PM concentration of 200 µg/mL and optimal exposure time of 24 hours were selected for subsequent experiments. Furthermore, a concentration-dependent increase of LDH release (Figure 1B) and ROS generation (Figure 1C,D) upon PM exposure was observed.
Effects of PM on the invasion of P. aeruginosa to the human bronchial epithelial cells
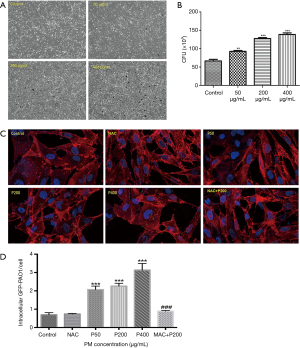
We first performed time-response experiments to determine the optimal infection time of P. aeruginosa. Infected with P. aeruginosa for 2 hours increased both bacterial adhesion and invasion to the airway epithelial cells without causing visible cytotoxicity, as assessed based on morphological changes and CFU assay (data not shown). However, a shorter infection time of 0.5 hour failed to cause bacterial adhesion and invasion to cells. Meanwhile, we failed to observe PM effects on P. aeruginosa invasion when BEAS-2B cells were infected with P. aeruginosa for longer time (4 or 6 hours), because it directly caused cell death. Thus, we chose 2 hours as our optimal time for the further bacteria invasion assays (Figure 2). PM was proved to significantly increased the number of bacterial invasion to the airway epithelial cells in concentration-dependent manner by CFU assay (Figure 2B), which is further confirmed by confocal microscopy (Figure 2C,D). Then BEAS-2B cells were pretreated with or without NAC (5 mM) for 1 h before PM (200 µg/mL) exposure. Twenty four hours later, cells were infected with P. aeruginosa (MOI 10) for an additional 2 hours. The result showed that the inhibition of ROS production significantly attenuated the PM-induced invasion of P. aeruginosa to BEAS-2B (Figure 2C,D), suggesting that ROS signaling pathway may be involved in the invasion process.
NAC attenuates PM-induced TJs disruption
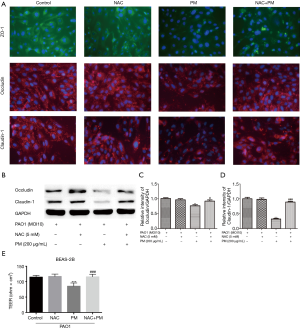
To further check whether ROS might mediate TJs proteins expressions, immunofluorescence and western blot were utilized to assess the expressions of ZO-1, occludin, claudin-1 and claudin-5. BEAS-2B did not express claudin-5 (data not shown). PM reduced the total protein abundance of occludin and claudin-1 at the cell membrane (Figure 3A,B,C,D). And the inhibition of ROS production significantly attenuated PM-induced ZO-1, occludin and claudin-1 disruptions at the cell membrane (Figure 3A). In addition, PM reduced TEER significantly, which was restored by NAC (Figure 3E).
PM increases lung injury, lung bacteria burden and blood bacterial dissemination accompanying by the degradation of the TJs in vivo
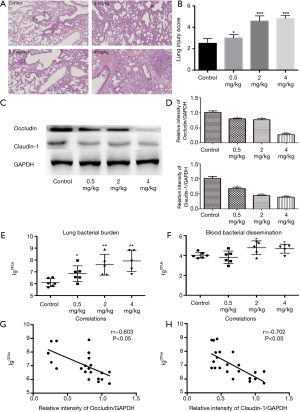
The results of lung histology showed that PM aggravated P. aeruginosa-infected lung injury in a concentration-dependent manner (Figure 4A,B). Compared to the control group, the protein expression of occludin and claudin-1 in PM (0.5, 2, or 4 mg/kg) exposed groups decreased significantly (Figure 4C,D), which were consistent with the in vitro results. The number of bacterial CFUs in the lungs and bloods had been analyzed as described in the materials and methods section. The number of CFUs detected in the lungs was significantly higher in the PM (0.5, 2, or 4 mg/kg) exposed mice compared to the control mice (Figure 4E). Bacterial CFUs of the blood in PM (0.5 mg/kg) exposed mice was similar to the control mice (Figure 4F). However, for mice exposed to 2 or 4 mg/kg of PM, the number of CFUs in the blood increased significantly compared to the control mice (Figure 4F), suggesting that PM increases the bacterial blood dissemination if the exposure concentration exceeds 2 mg/kg. In fact, an increase of the number of CFUs was closely correlated both with an increase in mice lung injury and with the degradation of TJs (Figure 4G,H), suggesting that a PM exposure in the airways contributes to an increasing lung injury, including the disruption of TJs which results in an increasing number of bacteria in the lung.
Discussion
PM is one of the most complex and harmful pollutants in the atmosphere, which has a wide range of sources and regional differences. In the real world, it is dispersed in all kinds of sizes. Thus, SRM 1649b containing different sizes and compositions was used to carry on this research. Different from the traditional point of view that considered the lower respiratory tract (LRT) as sterile, recent studies have demonstrated that there are lung microbiota colonizing in LRT both in healthy and in chronic respiratory diseases individuals (23-26). However, whether PM could promote opportunistic pathogens invasion to respiratory tract remains unclear. To investigate this point, the human bronchial epithelial cell lines (BEAS-2B cells) were chosen for our in vitro study.
In the study, we found that cell viability decreased in both concentration and time-dependent manner upon PM exposure, which were in accordance with previous studies (27,28). Furthermore, PM promoted the invasion of P. aeruginosa to airway epithelium, which was reversed by NAC, suggesting that the oxidative stress response was involved in this process. Then we observed that PM increased lung bacterial burden and blood bacteria dissemination in vivo model. Gally et al. (29) has reported that cigarette smoke exposure resulted in the decreased expression of the fatty acid binding protein 5 (FABP5), β defensin-2, and increased P. aeruginosa infection and inflammatory cytokine levels in primary normal human bronchial epithelial cells. Borcherding et al. (30) found that coal fly ash inhibited antimicrobial peptides activity and interfered with P. aeruginosa clearance in primary human airway epithelial culture model and in mice. Our results were basically consistent with these studies. However, the difference was that the PM used in our study is a well analyzed complex, which could properly imitate the real world exposure, rather than single source such as coal fly ash.
Furthermore, we assessed the effects of PM on the airway epithelial TJs protein including ZO-1, claudin-1 and occludin upon infection in both in vitro and in- in vivo model. We found that PM disrupted the TJs barrier, which may contribute to promote bacteria invasion into airway epithelia. It was already reported that the TJs play a key role in the regulation of paracellular transport (9). Without intact TJs structure, the epithelial barrier would not remain tight, allowing deposited PM to translocate across the barrier. Caraballo et al. (31) reported that PM breached alveolar epithelial barrier via the disruption of occludin. It might consequently promote PM to invade into the barrier. Wang et al. (32) demonstrated that PM disrupted endothelial cell barrier via ZO-1 degradation by activated calpain and thus resulted in vascular hyper-permeability (33). It was found in our study that PM decreased the total protein abundance of occludin and claudin-1 but not claudin-5 in the airway epithelia, which were consistent with previous study reporting that diesel exhaust particles exposure changed occludin distribution in airway epithelia (20) and PM exposure downregulated claudin-1 expression in human airway cells (14). But the major difference was that our study was performed in an infection model. In addition, oxidative stress has provided a vital mechanism for PM-induced cell damage (28). We observed that ROS induced by PM was closely related to PM-induced disruption of the claudin-1 and occludin proteins expressions in the airway epithelia and mice lung tissue. Most important, it was initially found that an increase of the number of CFUs was closely correlated to the reduced protein synthesis or increased protein degradation of the TJs, suggesting that a PM exposure in the airways contributes to an increasing lung injury, including the disruption of the TJs, then results in an increasing number of bacteria in the lung tissue. When lung tissue loses the ability to manipulate these TJs structures appropriately, it becomes more susceptible to various injuries, such as injury because of inhaled particles and bacterial infections.
However, there are some limitations in our study. Firstly, we could not exclude the role of PM-impaired immune cells such as neutrophils, NK cells and macrophages in promoting infection. Further study may be carried out to investigate it. Secondly, it was better to using an air-liquid model in the whole study for that it helps to keep the polarity of TJs. Third, although we used the 1649b containing different sizes and chemical constitutions to investigate the total effect of PM in order to imitate the real world condition, we still do not demonstrate exactly which kind of composition contributes more in this process.
Conclusions
We confirm that PM promotes P. aeruginosa invasion to airway epithelium and increases lung bacterial burden and blood bacterial dissemination. Inhibition of ROS production increases ZO-1, claudin-1 and occludin protein abundance to prevent PM-induced bacterial invasion. ZO-1, claudin-1 and occludin could be vital targets to regulate the airway epithelial permeability and defend bacterial invasion and blood bacterial dissemination upon PM exposure.
Acknowledgments
The authors gratefully acknowledge all members of the Yuanlin Song laboratory and Dr. Yaohui Li for their helpful discussions.
Funding: This work was supported by the State Key Basic Research Program project (2015CB553404), the National Natural Science Foundation of China key grant (81630001, 81490533), grant (81770075, 81500026, 81570028, 81600056), Shanghai Science and Technology Committee grant (15DZ1930600/15DZ1930602/16ZR1405700) and Shanghai Municipal Commission of Health and Family Planning (201540370).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All protocols for animal experiments were approved by the Animal Care and Use Committee of Zhongshan Hospital, Fudan University.
References
- Falcon-Rodriguez CI, Osornio-Vargas AR, Sada-Ovalle I, et al. Aeroparticles, Composition, and Lung Diseases. Front Immunol 2016;7:3. [Crossref] [PubMed]
- Mohammed MOA, Song WW, Ma WL, et al. Potential Toxicological and Cardiopulmonary Effects of PM2.5 Exposure and Related Mortality: Findings of Recent Studies Published during 2003-2013. Biomed Environ Sci 2016;29:66-79. [PubMed]
- Sigaud S, Goldsmith CAW, Zhou HW, et al. Air pollution particles diminish bacterial clearance in the primed lungs of mice. Toxicol Appl Pharmacol 2007;223:1-9. [Crossref] [PubMed]
- Zhao H, Li WX, Gao YF, et al. Exposure to particular matter increases susceptibility to respiratory Staphylococcus aureus infection in rats via reducing pulmonary natural killer cells. Toxicology 2014;325:180-8. [Crossref] [PubMed]
- Martí-Lliteras P, Regueiro V, Morey P, et al. Nontypeable Haemophilus influenzae Clearance by Alveolar Macrophages Is Impaired by Exposure to Cigarette Smoke. Infect Immun 2009;77:4232-42. [Crossref] [PubMed]
- Clark ML, Bazemore H, Reynolds SJ, et al. A Baseline Evaluation of Traditional Cook Stove Smoke Exposures and Indicators of Cardiovascular and Respiratory Health among Nicaraguan Women. Int J Occup Environ Health 2011;17:113-21. [Crossref] [PubMed]
- Williams BJ, Dehnbostel J, Blackwell TS. Pseudomonas aeruginosa: Host defence in lung diseases. Respirology 2010;15:1037-56. [Crossref] [PubMed]
- Psoter KJ, De Roos AJ, Mayer JD, et al. Fine particulate matter exposure and initial Pseudomonas aeruginosa acquisition in cystic fibrosis. Ann Am Thorac Soc 2015;12:385-91. [Crossref] [PubMed]
- Schlingmann B, Molina SA, Koval M. Claudins: Gatekeepers of lung epithelial function. Semin Cell Dev Biol 2015;42:47-57. [Crossref] [PubMed]
- Günzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol Rev 2013;93:525-69. [Crossref] [PubMed]
- Suzuki H, Tani K, Tamura A, et al. Model for the architecture of claudin-based paracellular ion channels through tight junctions. J Mol Biol 2015;427:291-7. [Crossref] [PubMed]
- Kaarteenaho R, Merikallio H, Lehtonen S, et al. Divergent expression of claudin -1, -3, -4, -5 and -7 in developing human lung. Respir Res 2010;11:59. [Crossref] [PubMed]
- Runkle EA, Rice SJ, Qi J, et al. Occludin is a direct target of thyroid transcription factor-1 (TTF-1/NKX2-1). J Biol Chem 2012;287:28790-801. [Crossref] [PubMed]
- Kim SS, Kim CH, Kim JW, et al. Airborne particulate matter increases MUC5AC expression by downregulating Claudin-1 expression in human airway cells. BMB Rep 2017;50:516-21. [Crossref] [PubMed]
- Nomura K, Obata K, Keira T, et al. Pseudomonas aeruginosa elastase causes transient disruption of tight junctions and downregulation of PAR-2 in human nasal epithelial cells. Respir Res 2014;15:21. [Crossref] [PubMed]
- Jin W, Rong L, Liu Y, et al. Increased claudin-3, -4 and -18 levels in bronchoalveolar lavage fluid reflect severity of acute lung injury. Respirology 2013;18:643-51. [Crossref] [PubMed]
- Zaas DW, Duncan MJ, Li GJ, et al. Pseudomonas invasion of type I pneumocytes is dependent on the expression and phosphorylation of caveolin-2. Journal of Biological Chemistry 2005;280:4864-72. [Crossref] [PubMed]
- Wu HM, Wang J, Zhang B, et al. CpG-ODN promotes phagocytosis and autophagy through JNK/P38 signal pathway in Staphylococcus aureus-stimulated macrophage. Life Sci 2016;161:51-9. [Crossref] [PubMed]
- Song Y, Baer M, Srinivasan R, et al. PcrV antibody-antibiotic combination improves survival in Pseudomonas aeruginosa-infected mice. Eur J Clin Microbiol Infect Dis 2012;31:1837-45. [Crossref] [PubMed]
- Lehmann AD, Blank F, Baum O, et al. Diesel exhaust particles modulate the tight junction protein occludin in lung cells in vitro. Part Fibre Toxicol 2009;6:26. [Crossref] [PubMed]
- Han X, Liang WL, Zhang Y, et al. Effect of atmospheric fine particles on epidermal growth factor receptor mRNA expression in mouse skin tissue. Genet Mol Res 2016. [Crossref] [PubMed]
- Sawa T, Yahr TL, Ohara M, et al. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat Med 1999;5:392-8. [Crossref] [PubMed]
- Dickson RP, Erb-Downward JR, Freeman CM, et al. Spatial Variation in the Healthy Human Lung Microbiome and the Adapted Island Model of Lung Biogeography. Ann Am Thorac Soc 2015;12:821-30. [Crossref] [PubMed]
- Bassis CM, Erb-Downward JR, Dickson RP, et al. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. MBio 2015;6:e00037. [Crossref] [PubMed]
- Marsh RL, Kaestli M, Chang AB, et al. The microbiota in bronchoalveolar lavage from young children with chronic lung disease includes taxa present in both the oropharynx and nasopharynx. Microbiome 2016;4:37. [Crossref] [PubMed]
- Man WH, de Steenhuijsen Piters WA, Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol 2017;15:259-70. [Crossref] [PubMed]
- Naimabadi A, Ghadiri A, Idani E, et al. Chemical composition of PM10 and its in vitro toxicological impacts on lung cells during the Middle Eastern Dust (MED) storms in Ahvaz, Iran. Environ Pollut 2016;211:316-24. [Crossref] [PubMed]
- Yang GZ, Wang ZJ, Bai F, et al. Epigallocatechin-3-gallate protects HUVECs from PM2.5-induced oxidative stress injury by activating critical antioxidant pathways. Molecules 2015;20:6626-39. [Crossref] [PubMed]
- Gally F, Chu HW, Bowler RP. Cigarette smoke decreases airway epithelial FABP5 expression and promotes Pseudomonas aeruginosa infection. PLoS One 2013;8:e51784. [Crossref] [PubMed]
- Borcherding JA, Chen H, Caraballo JC, et al. Coal fly ash impairs airway antimicrobial peptides and increases bacterial growth. PLoS One 2013;8:e57673. [Crossref] [PubMed]
- Caraballo JC, Yshii C, Westphal W, et al. Ambient particulate matter affects occludin distribution and increases alveolar transepithelial electrical conductance. Respirology 2011;16:340-9. [Crossref] [PubMed]
- Wang T, Wang L, Moreno-Vinasco L, et al. Particulate matter air pollution disrupts endothelial cell barrier via calpain-mediated tight junction protein degradation. Part Fibre Toxicol 2012;9:35. [Crossref] [PubMed]
- Wang T, Chiang ET, Moreno-Vinasco L, et al. Particulate matter disrupts human lung endothelial barrier integrity via ROS- and p38 MAPK-dependent pathways. Am J Respir Cell Mol Biol 2010;42:442-9. [Crossref] [PubMed]


