Does oxidative stress contribute to antituberculosis drug resistance?
Escalation of drug resistance in Mycobacterium tuberculosis (MTB) furnishes a formidable challenge to the global control of tuberculosis (TB) (1). Delineation of the underlying mechanisms of anti-TB drug resistance is germane to the discovery of strategies in tackling this worldwide health care crisis. Aside from the development of new anti-TB drugs to circumvent the drug resistance scenarios to improve patient treatment outcomes, it appears pertinent to explore whether modulation of host factors or responses could help to ameliorate the probability of drug-resistant TB.
There is quite some evidence that rifampicin and isoniazid could produce oxidative burst in their antimicrobial mechanisms (2-4). Similarly, fluoroquinolones have also been shown to display such a phenomenon (5). In addition to the antioxidative capacity mounted by MTB to counteract the (bactericidal) oxidative burst associated with anti-TB drug challenge, development of a metabolically quiescent bacillary persister state also helps the challenged MTB to survive within host macrophages and tissue biofilms (6).
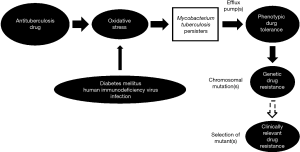
Bacterial persisters putatively arise in a stochastic manner, with influence from stress-inducible quorum sensing (7,8). MTB is well known to form persisters that play an important role in persistent infection requiring prolonged treatment (9). In recent years, there has been a surge in reports regarding the induction of bacterial persisters, including those of MTB by oxidative/nitrosative stress (or more broadly disturbance in redox homeostasis) (10-14). Upregulation or activation of efflux pump(s) that result in extrusion of substrates and drugs leading to low-level resistance (often referred as phenotypic tolerance) against anti-TB agents can operate in MTB persisters (15-17). It is conceivable that phenotypic tolerance does not usually occur in isolation, as revealed by accumulating experimental evidence (16,18,19). This initiation step is apparently of crucial importance in the march of bacillary resistance against anti-TB drugs (Figure 1). Phenotypic tolerance facilitates the development of genetic resistance in MTB that generally results from spontaneous chromosomal mutations, eventually leading to higher levels of resistance to anti-TB agents. While the exact triggers for such facilitation do not appear to be totally clear (20), fluctuation in drug levels, especially in the presence of those comorbidities of TB with oxidative stress inherent to their disease pathogenesis, might contribute. Diabetes mellitus and HIV infection, the two major comorbidities to TB globally are noted to have poorer TB treatment outcomes, inclusive of lower success, higher relapse and increased propensity for development of drug resistance. The likely underlying mechanism has been hypothesized to be the induction of MTB persisters, as a core dormancy response to the oxidative stress furnished by the various players (Figure 1) (10,21). Importantly, both diabetes mellitus and HIV infection can be associated with malabsorption states with reduced drug bioavailability, resulting in erratic anti-TB drug levels (10,21). The afore-mentioned conceptual model regarding the development of antituberculosis drug resistance is a simplistic one. Further research is required to understand the complex underlying mechanisms better.
Clinically relevant drug-resistant TB adversely impacting treatment outcomes occur as a result of selection of the genetic mutants through man-made blunders that often exist in a poorly implemented TB programme, involving mainly doctors, administrators and patients (22). Suboptimal prescription (regarding drug dosage and drug scheduling) and inadequate drug supply, as well as poor treatment adherence are conducive to the drug resistance scenarios currently prevailing in many parts of the world.
The contributing role of oxidative stress, alongside its interaction with immunological dysfunction, to antituberculosis drug resistance merits further unravelling through translational research and clinical research. Amelioration of oxidative stress might conceivably help in preempting initiation of the march of drug resistance. Optimal metabolic control in diabetic patients can reduce oxidative stress, and so does better control of the viral load in HIV infection (10,21). Advancement in pharmacotherapy for HIV infection to circumvent inadvertent heightening of oxidative stress during antiviral therapy is likely beneficial (21,23). Furthermore, delineation of the role of antioxidants and other associated therapeutics as adjunctive strategy may be warranted (24). A potentially interesting example is vitamin C with both prooxidant and antioxidant activities that may help to enhance antituberculosis drug efficacy and reduce the risk of antituberculosis drug resistance (25,26). Last but not least, halting the march of anti-TB drug resistance mandates strengthening of the infrastructure of TB programmes to forestall the development of clinically relevant drug-resistant TB in many countries (27-29).
Acknowledgments
None.
Footnote
Conflicts of Interest: Dr. WW Yew was consultant to Otsuka Pharmaceutical Company until July 2016. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- World Health Organization. Global tuberculosis report 2018. World Health Organization; 2018.
- Piccaro G, Pietraforte D, Giannoni F, et al. Rifampin induces hydroxyl radical formation in Mycobacterium tuberculosis. Antimicrob Agents Chemother 2014;58:7527-33. [Crossref] [PubMed]
- Sharma R, Muttil P, Yadav AB, et al. Uptake of inhalable microparticles affects defence responses of macrophages infected with Mycobacterium tuberculosis H37Ra. J Antimicrob Chemother 2007;59:499-506. [Crossref] [PubMed]
- Shetty A, Dick T. Mycobacterial cell wall synthesis inhibitors cause lethal ATP burst. Front Microbiol 2018;9:1898. [Crossref] [PubMed]
- Gurumurthy M, Rao M, Mukherjee T, et al. A novel F(420)-dependent anti-oxidant mechanism protects Mycobacterium tuberculosis against oxidative stress and bactericidal agents. Mol Microbiol 2013;87:744-55. [Crossref] [PubMed]
- Shastri MD, Shukla SD, Chong WC, et al. Role of oxidative stress in the pathology and management of human tuberculosis. Oxid Med Cell Longev 2018;2018:7695364. [Crossref] [PubMed]
- Michiels JE, Van den Bergh B, Verstraeten N, et al. Molecular mechanisms and clinical implications of bacterial persistence. Drug Resist Updat 2016;29:76-89. [Crossref] [PubMed]
- Zhang Y. Persisters, persistent infections and the Yin-Yang model. Emerg Microbes Infect 2014;3:e3. [Crossref] [PubMed]
- Zhang Y, Yew WW, Barer MR. Targeting persisters for tuberculosis control. Antimicrob Agents Chemother 2012;56:2223-30. [Crossref] [PubMed]
- Yew WW, Leung CC, Zhang Y. Oxidative stress and TB outcomes in patients with diabetes mellitus? J Antimicrob Chemother 2017;72:1552-5. [Crossref] [PubMed]
- Vijay S, Hai HT, Thu DD, et al. Ultrastructural analysis of cell envelope and accumulation of lipid inclusions in clinical Mycobacterium tuberculosis isolates from sputum, oxidative stress, and iron deficiency. Front Microbiol 2018;8:2681. [Crossref] [PubMed]
- Kumar A, Farhana A, Guidry L, et al. Redox homeostasis in mycobacteria: the key to tuberculosis control? Expert Rev Mol Med 2011;13:e39. [Crossref] [PubMed]
- Lamichhane G. Mycobacterium tuberculosis response to stress from reactive oxygen and nitrogen species. Front Microbiol 2011;2:176. [Crossref] [PubMed]
- Mehta M, Singh A. Mycobacterium tuberculosis WhiB3 maintains redox homeostasis and survival in response to reactive oxygen and nitrogen species. Free Radic Biol Med 2019;131:50-8. [Crossref] [PubMed]
- Ramón-García S, Martín C, Thompson CJ, et al. Role of the Mycobacterium tuberculosis P55 efflux pump in intrinsic drug resistance, oxidative stress responses, and growth. Antimicrob Agents Chemother 2009;53:3675-82. [Crossref] [PubMed]
- Schmalstieg AM, Srivastava S, Belkaya S, et al. The antibiotic resistance arrow of time: efflux pump induction is a general first step in the evolution of mycobacterial drug resistance. Antimicrob Agents Chemother 2012;56:4806-15. [Crossref] [PubMed]
- Chen C, Gardete S, Jansen RS, et al. Verapamil targets membrane energetics in Mycobacterium tuberculosis. Antimicrob Agents Chemother 2018;62:e02107-17. [Crossref] [PubMed]
- den Hertog AL, Menting S, van Soolingen D, et al. Mycobacterium tuberculosis Beijing genotype resistance to transient rifampin exposure. Emerg Infect Dis 2014;20:1932-3. [Crossref] [PubMed]
- Sebastian J, Swaminath S, Nair RR, et al. De novo emergence of genetically resistant mutants of Mycobacterium tuberculosis from the persistence phase cells formed against antituberculosis drugs in vitro. Antimicrob Agents Chemother 2017;61. [Crossref] [PubMed]
- Pasipanodya JG, Gumbo T. A new evolutionary and pharmacokinetic-pharmacodynamic scenario for rapid emergence of resistance to single and multiple anti-tuberculosis drugs. Curr Opin Pharmacol 2011;11:457-63. [Crossref] [PubMed]
- Yew WW, Chan DP, Singhal A, et al. Does oxidative stress contribute to adverse outcomes in HIV-associated TB? J Antimicrob Chemother 2018;73:1117-20. [Crossref] [PubMed]
- Zhang Y, Yew WW. Mechanisms of drug resistance in Mycobacterium tuberculosis. Int J Tuberc Lung Dis 2009;13:1320-30. [PubMed]
- Sharma B. Oxidative stress in HIV patients receiving antiretroviral therapy. Curr HIV Res 2014;12:13-21. [Crossref] [PubMed]
- Yew WW, Chang KC, Chan DP, et al. Can modulating redox status help to enhance antituberculosis drug efficacy? Tuberculosis (Edinb) 2018;113:177-8. [Crossref] [PubMed]
- Yew WW, Chang KC, Leung CC, et al. Vitamin C and Mycobacterium tuberculosis persisters. Antimicrob Agents Chemother 2018;62. [Crossref] [PubMed]
- Yew WW, Chang KC, Chan DP, et al. Can vitamin C help in managing tuberculosis associated with diabetes mellitus? Respirology 2019;24:819-20. [Crossref] [PubMed]
- Khan MS, Hutchison C, Coker RJ. Risk factors that may be driving the emergence of drug resistance in tuberculosis patients treated in Yangon, Myanmar. PLoS One 2017;12:e0177999. [Crossref] [PubMed]
- Mehta S, Yu EA, Ahamed SF, et al. Rifampin resistance and diabetes mellitus in a cross-sectional study of adult patients in rural South India. BMC Infect Dis 2015;15:451. [Crossref] [PubMed]
- Leung CC, Yew WW, Mok TY, et al. Effects of diabetes mellitus on the clinical presentation and treatment response in tuberculosis. Respirology 2017;22:1225-32. [Crossref] [PubMed]