
House dust mite subcutaneous immunotherapy in Chinese patients with allergic asthma and rhinitis
Introduction
The best strategy for treating allergic diseases is a combination of approaches including avoiding allergens, patient education, appropriate drug therapy, and allergen immunotherapy (AIT). AIT is recommended by Global Strategy for Asthma Management and Prevention (GINA) for a treatment option if allergy play a prominent role, e.g., asthma with allergic rhinoconjunctivitis (1). Currently, two types of AIT are mainly applied in clinical practice: subcutaneous immunotherapy (SCIT) is conventionally administered by the subcutaneous route, and sublingual immunotherapy (SLIT) is a new immunotherapy by sublingual administration of soluble allergen extracts (2,3). The efficacy of SCIT and SLIT for both children and adults for pollen, pet dander, and house dust mite (HDM) has been confirmed in recent systematic meta-analyses (4,5). However, the exact mechanism underlying the therapeutic effect of AIT is still unclear, and the clinical practice of immunotherapy in China has yet to be standardized. The key issue in this review is to optimize the therapeutic effect of AIT, confirm that it truly changes the patient’s allergic status, and maintain a long-term efficacy.
Exposure to HDM allergen is a major cause of perennial allergic rhinitis (AR) and/or asthma in China, predominantly Dermatophagoides pteronyssinus (Der p) and Dermatophagoides farina (Der f) (6). Since 2001, SCIT in the form of an HDM vaccine has been widely used in China. This review summarizes the clinical efficacy of SCIT and its possible underline mechanisms on allergic asthma and/or rhinitis.
Symptom and medication scores
All reports have confirmed that SCIT significantly alleviated the symptoms of AR and/or allergic asthma and reduced the dosage of other medications. A total of 129 patients completed a multicenter, randomized, double-blinded, placebo-controlled 1-year study of mild to moderate allergic asthma conducted by Wang et al. (7). In this study, subjects between the ages of 6 and 45 years who fulfilled the GINA guidelines for stabilized mild to moderate asthma with their lung function of forced expiratory volume in 1 second ≥70% of predicted (8), and had positive skin prick test (SPT) and specific immunoglobulin E (sIgE) to Der p were included. Subjects were excluded with positive SPT to animals and pets at home. Percentage of the subjects accompanied with rhinitis in active and placebo were 90.6% and 84.6% respectively without significant difference. During the study, subjects were asked to rate each of the daytime symptoms of shortness of breath, wheeze, cough and chest tightness from 0 to 5. The mean of the four scores was recorded as the daytime symptom score. Night-time symptoms were scored from 0 to 4 according to the frequency of nocturnal and early morning awakening by asthma. The daily symptom score was the sum of the daytime and night-time symptom scores. The medication score is calculated by assigning a score of 1 to each puff of salbutamol/terbutaline or the equivalent dose of oral β2-agonist. Just prior to unblinding, subjects were asked to give self-evaluations in their improvement in exacerbation frequency, exacerbation severity and overall symptoms, based on their own impression. The authors noted significant differences between the treated and control groups with respect to asthma symptom scores, starting from 7 months of treatment. In the treated group, the asthma symptom and medication scores of the patients were significantly lower in maintenance phase than those in up-dosing phase. After 1 year, the self-evaluations of improvements were significantly better in the treated group than in the control group. The authors also analyzed the effect of inhaled corticosteroids (ICS) on asthmatic symptoms and the usage of as needed inhaled and oral short-acting β2-agonists. Their results showed that in patients who were already using ICS with constant dose during the treatment period, SCIT significantly improved their asthmatic symptoms and reduced their need for as needed β2-agonists; in patients who did not start to use ICS before the study, although SCIT did not significantly improve their asthmatic symptoms, it reduced their need for rescued β2-agonists. These results indicated that SCIT can relieve the symptoms and reduce the need for medications in asthmatic patients. The authors also followed 38 patients who received SCIT for 2–3 years in one of the centers, and they found that SCIT continued to improve asthma symptoms and reduce the medication doses.
Qi et al. (9) reported early intervention with SCIT helps to improve the efficacy of AR treatment and local reactions might predict successful SCIT. Zhang et al. (10) treated 154 patients with moderate to severe persistent AR using a cluster SCIT schedule and followed them for 1 year. They showed the rhinitis scores significantly decreased starting from the 4th week after treatment, which was earlier than using the conventional injection regimen. The cluster schedule also reduced the duration of up-dosing phase by over 60% as compared to the conventional schedule and improved treatment compliance. Rhinitis and medication scores of the patients significantly decreased after 1 year, and scores for quality of life of patients with rhinitis significantly increased, indicating a significant improvement of overall life quality. Feng et al. (11) compared the combined symptom and medication (including inhaled and nasal corticosteroid, inhaled short acting β2 agonists, and oral antihistamine) scores (SMS) between SCIT patients and only-medication-treated patients, and found that SCIT patients had a significant improvement in SMS from weeks 52 to 156 compared with medication-treated control subjects. Lai et al. (12) also reported that the symptom and medication scores of asthma in children and adults reduced significantly after 25 weeks of SCIT while there were no significant differences between children and adults during the whole course (Table 1).
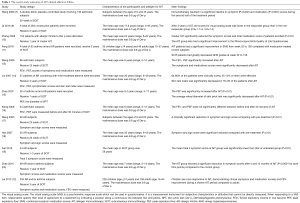
Full table
Pulmonary function and airway hyperresponsiveness
The responses of pulmonary functions to SCIT are different with different studies. Wang et al. (7) reported the pulmonary functions of the patients in treated group did not change after 1 year of treatment, and the variations in peak expiratory flow (PEF) and peak expiratory flow rate (PEFR) in the morning versus evening did not show significant differences between active SCIT and placebo patients. The improvement in non-specific airway hyperresponsiveness was also comparable between the two groups. These results may be related to the regular usage of ICS in most of the patients included in the study. However, after the patients were given 2–3 years of SCIT treatment, their non-specific airway hyperresponsiveness gradually decreased, even returning to normal in some patients. This result indicated that the amelioration of airway inflammation may take longer than symptom improvement, and it explained why SCIT must be taken for 3–5 years. One study by Wang et al. (13) showed that after 25 weeks treatment, pulmonary function indexes of pediatric patients with asthma were significantly improved.
Specific allergen sensitivity
Many studies have demonstrated reductions of skin responses to allergen prick test used to assess sIgE-mediated immediate allergic reactions. Wang et al. (13) showed skin indexes (SI) to HDM after 1 year were significantly reduced in patients treated with SCIT than those with placebos. After 2–3 years, in patients with SCIT, SI was further decreased and significantly smaller than that before treatment, but serum HDM sIgE and eosinophil cationic protein (ECP) did not decrease after 3 years of treatment. Lin et al. (14) reported that after the patients were treated for 3 years, their serum HDM sIgE showed a trend towards decreasing but without statistical significance, while ECP was significantly decreased. Zhao et al. (15) also demonstrated a significant decrease in the average wheal diameter of SPT against HDM after the patients were treated with SCIT for 1 year.
Safety evaluation
In Chinese Guideline on AIT for AR (8), Bao et al. compared the classifications of systemic reactions between guidelines from the European Academy of Allergy and Clinical Immunology (EAACI) (21) and World Allergy Organization (WAO) (22) for AIT and summarized them in five grades, of which Grade 0 with no symptoms or nonspecific symptoms, Grade I with mild systemic reactions, Grade II with moderate systemic reactions, Grade III with severe (non-life-threatening) systemic reactions, Grade IV with anaphylactic shock that symptoms/signs presenting in more than one organ or system, and Grade V with severe systemic reactions causing death. According to the results of studies performed in other countries, the prevalence of systemic adverse reactions ranges from 3.7% to 5.2% in all patients who had received SCIT, which is about 0.093% to 0.3% of the total number of injections (23), and the incidence of severe fatal systemic adverse reactions is one event per 1 to 2.5 million injections (24-26). In a study reported by Zeng et al. (27), among 15,645 injections, immediate local and systemic adverse reactions occurred in 8,523 (54.5%) and 397 (2.5%) injections respectively, this result is in accordance with the literatures. Most of the reactions occurred within 30 min of the injection. The symptoms were mild and the responses to treatment were good. No serious adverse reaction was noted. Since the incidence of systemic adverse reactions is higher during the up-dosing phase than the maintenance phase, Xiang et al. (16) studied the tolerances to the allergen vaccine of SCIT in 24 pediatric patients with mild to moderate allergic asthma during the up-dosing phase. They found that the incidence of systemic adverse reaction was 3.7%. Most of these were late reactions and all of which were Grade II reactions. The incidence of local adverse reactions was 14.9%, and most were mild. Local reactions showed no predictive values for systemic reactions. A study by Wang et al. (13) revealed an incidence of 0.7% for systemic adverse effect in pediatric patients, indicating the allergen vaccine is of low rate of reactions for the treatment of pediatric allergic asthma.
In the studies by Zeng et al. (27), Wang et al. (17), Han et al. (18), and Liu et al. (28), the incidence of systemic adverse reactions was 0.57–2.4% for a conventional injection schedule used to treat AR. Zhang et al. (10) administered a total of 3,464 injections to AR patients with the cluster injection schedule. The incidence of systemic adverse reactions was 5.9% and 0.75%, respectively, for the total number of patients and injections. This result is slightly higher than reported. Most of the systemic adverse reactions occurred during the up-dosing phase, which accounted for 3.9% and 0.71%, respectively, of the total number of patients and injections. The prevalence of Grade III reactions that required adrenaline treatment was around 0.12% of the total injections (17,18,27,28). In accordance to studies performed in other countries, no Grade IV reaction occurred. Most of the adverse reactions occurred in patients with asthma. Therefore, asthma is the main risk factor of systemic adverse reaction to SCIT. According to guidelines from China, EAACI and WAO, SCIT cannot be given in patients with unstable asthma or FEV1 lower than 70% of the expected value. All patients reported with systemic adverse reactions achieved rapid remission when treated and no hospitalization was required, indicating that SCIT is safe both in conventional and cluster schedule (Table 2). Nevertheless, GINA guideline emphasizes that AIT must be weighed against the risk of side effects in patients with asthma (1). EAACI position paper (21) gives the instruction that the scheduled allergen dose should be reduced in case of a systemic reaction at the preceding visit. The magnitude of reduction depends on the severity of the reaction. In case of anaphylactic and other life-threatening reactions the continuation of SCIT should be carefully evaluated.
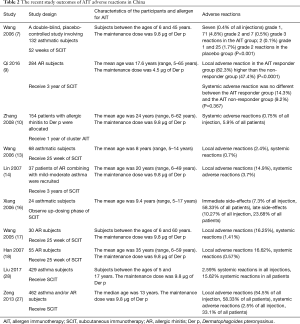
Full table
Mechanisms underlying SCIT
The mechanisms that are associated with AIT involve antibody responses and cellular reactions. Wang et al. (29) evaluated T helper cell-secreted cytokines and DNA methylation patterns in children treated with Der p AIT, they found decreased IL-2 production and increased IL-4 cytokine promoter methylation. Fan et al. (19) investigated the response of group 2 innate lymphoid cells (ILC2s) of peripheral blood in HDM-sensitized Chinese patients with AR who received SCIT with Der p extract, they found the levels of ILC2s in the peripheral blood of immunotherapy group were significantly reduced compared with that in untreated group, and suggested that the relatively high level of ILC2s in AR patients sensitized to HDM may be treated by Der p SCIT, and a reduction of ILC2 levels might contribute to symptom remission and immunologic tolerance in AR. Zhao et al. (20) found that IgG4 and IgE blocking factor correlated with symptoms at 12 months of SCIT, where the assay of IgE blocking factor measured the blocking activity of IgE and allergen binding. Feng et al. (11) found that Der p sIgG4 in SCIT patients significant increased from week 12 to 156, and serum obtained from SCIT patients significantly inhibited Der p sIgE binding to B cells after 16 weeks. Luo et al. (30) found microRNAs and Foxp3 mRNA was significantly increased after 3 months of SCIT and SLIT in children with persistent AR. Zeng et al. (31) investigated the serum specifics IgE and sIgG4 to allergen component of Der p, they found the Der p 1 and Der p 2 sIgE levels were elevated at 6 months and progressively declined from 12 months, and the sIgG4 levels for Der p, Der p 1 and Der p 2 were increasing during the first year and reached a plateau thereafter. Lai et al. (12) found the levels of Der p-specific IgG4 significant increase after 10 weeks of subcutaneous AIT, and the increase ratio of Der p-specific IgG4 was higher in children than adults at 52 weeks and 104 weeks of AIT (Table 3).
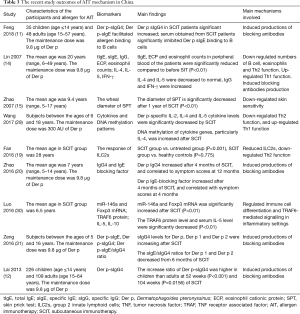
Full table
Conclusions and practical considerations
A 1- to 3-year administration of the standardized subcutaneous injection of HDM vaccine as recommended by the GINA, EAACI, WAO and China guidelines, in patients with AR and mild to moderate allergic asthma can significantly improve rhinitis and asthma symptoms, reduce the need for medication, and decrease skin sensitivity to HDM. SCIT can also maintain stable pulmonary functions in all patients, improve pulmonary function in pediatric patients and reduce non-specific airway hyperresponsiveness. The safety of this allergen vaccine in Chinese patients is similar to that reported in foreign studies. Successful AIT may induce antibody responses and cellular reactions. The studies of AIT in China suggest AIT with standardized allergen vaccine can ensure the efficacy and safety in patients with allergic diseases when the guidelines are closely followed and the indications and contradictions of treatment are adhered carefully.
Among current AR and asthma therapies, AIT is the only therapy that can change the underlying natural history of the allergic conditions. However, before the physicians decide to apply AIT to patients, the following considerations must be taken into account (8,21,22):
- Clinical symptoms of the patients are induced predominantly by allergen exposure;
- Using standardized products with documented clinical efficacy and safety is strongly recommended;
- AIT should be prescribed only by an allergist-immunologist or other physician who is expertly trained in the therapy;
- AIT should be administered under the supervision of an allergist-immunologist or other physician specifically trained in immunotherapy, the early signs and symptoms of anaphylaxis, and appropriate emergency procedures and medications;
- SCIT should be given only in facilities equipped to treat anaphylaxis;
- The health status of the patient should be evaluated prior to every injection. Patients who are acutely ill, especially with asthma or respiratory difficulties, should not receive immunotherapy until their disease is stabilized;
- Patients should always be asked about current medications prior to immunotherapy, to avoid interactions with beta blockers and other conflicting medications;
- Patients must wait at the health care facility a minimum of 30 min after an allergen injection. The time period may be extended for high-risk patients.
Acknowledgments
Funding: This study was funded by the Breeding Program of Major Research Plan (91542104) of the National Natural Science Foundation of China, and Precision Medicine Research of The National Key Research and Development Plan of China (2016YFC0905800) to J Li, and supported by the National Natural Science Foundation of China (81500024) to M Feng.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Revised, 2018. Available online: https://ginasthma.org/
- Matsuoka T, Shamji MH, Durham SR. Allergen immunotherapy and tolerance. Allergol Int 2013;62:403-13. [Crossref] [PubMed]
- Jutel M, Akdis CA. Novel immunotherapy vaccine development. Curr Opin Allergy Clin Immunol 2014;14:557-63. [Crossref] [PubMed]
- Casale TB, Stokes JR. Immunotherapy: what lies beyond. J Allergy Clin Immunol 2014;133:612-9. [Crossref] [PubMed]
- Soyka MB, van de Veen W, Holzmann D, et al. Scientific foundations of allergen-specific immunotherapy for allergic disease. Chest 2014;146:1347-57. [Crossref] [PubMed]
- Li J, Sun B, Huang Y, et al. China Alliance of Research on Respiratory Allergic Disease (CARRAD) A multicenter study assessing the prevalence of sensitizations in patients with asthma and/or rhinitis in China. Allergy 2009;64:1083-92. [Crossref] [PubMed]
- Wang H, Lin X, Hao C, et al. A double-blind, placebo-controlled study of house dust mite immunotherapy in Chinese asthmatic patients. Allergy 2006;61:191-7. [Crossref] [PubMed]
- Bao Y, Chen J, Cheng L, et al. Chinese Society of Allergy(CSA) and Chinese Allergic Rhinitis Collaborative Research Group (C2AR2G).Chinese Guideline on allergen immunotherapy for allergic rhinitis. J Thorac Dis 2017;9:4607-50. [Crossref] [PubMed]
- Qi S, Chen H, Huang N, et al. Early Intervention Improves Clinical Responses to House Dust Mite Immunotherapy in Allergic Rhinitis Patients. Int Arch Allergy Immunol 2016;171:234-40. [Crossref] [PubMed]
- Zhang L, Wang CS, Wang XD, et al. Efficacyand safety of cluster immunotherapy for 154 patients with allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2008;43:187-91. [PubMed]
- Feng M, Su Q, Lai X, et al. Functional and Immunoreactive Levels of IgG4 Correlate with Clinical Responses during the Maintenance Phase of House Dust Mite Immunotherapy. J Immunol 2018;200:3897-904. [Crossref] [PubMed]
- Lai X, Li J, Xiao X, et al. Specific IgG4 production during house dust mite immunotherapy among age, gender and allergic disease populations. Int Arch Allergy Immunol 2013;160:37-46. [Crossref] [PubMed]
- Wang MK, Huang Y, Liu EM, et al. Standardized mites allergens immunotherapy for allergic asthma in 68 children. Chinese Journal of New Drugs and Clinical Remedies 2006;25:935-8.
- Lin XP, Gao J, Zheng Y, et al. Efficacy evaluation of specific immunotherapy with standardization allergen vaccine of dermatophagoides pteronyssinus. Journal of Clinical Otorhinolaryngology Head, and Neck Surgery 2007;14:7-10.
- Zhao GJ, Guo HX, Li YQ. Standardized allergens immunotherapy for allergic asthma in 30 children. Central Plains Medical Journal 2007;34:64-5.
- Xiang L, Shen KL, Zhang HY, et al. Tolerance during dose-increase phase of specific immunotherapy with standardized house-dust mite extract in asthmatic children. Chin J Prac Pediatr 2006;21:924-6.
- Wang CS, Zhang L, Han DM. Primary evaluation of the efficacy and safety of standardized dust mite allergen vaccine in the treatment of allergic rhinitis. Journal of Capital University of Medical Sciences 2005;26:246-8.
- Han H, Chen SH, Qiu QH. Efficacy evaluation of specific immunotherapy with standardization allergen vaccine in allergic rhinitis patients. Guangdong Medical Journal 2007;28:991-2.
- Fan DC, Wang XD, Wang CS, et al. Suppression of immunotherapy on Group 2 innate lymphoid cells in allergic rhinitis. Chin Med J (Engl) 2016;129:2824-8. [Crossref] [PubMed]
- Zhao D, Lai X, Tian M, et al. The functional IgE-blocking factor induced by allergen-specific immunotherapy correlates with IgG4 antibodies and a decrease of symptoms in house dust mite-allergic children. Int Arch Allergy Immunol 2016;169:113-20. [Crossref] [PubMed]
- Alvarez-Cuesta E, Bousquet J, Canonica GW, et al. Standards for practical allergen-specific immunotherapy. Allergy 2006;61 Suppl 82:1-20. [Crossref] [PubMed]
- Cox L, Larenas-Linnemann D, Lockey RF, et al. Speaking the same language: The World Allergy Organization Subcutaneous Immunotherapy Systemic Reaction Grading System. J Allergy Clin Immunol 2010;125:569-74. [Crossref] [PubMed]
- Bernstein DI, Wanner M, Borish L, et al. Immunotherapy Committee AAoAA, Immunology. Twelve-year survey of fatal reactions to allergen injections and skin testing:1990-2001. J Allergy Clin Immunol 2004;113:1129-36. [Crossref] [PubMed]
- Amin HS, Liss GM, Bernstein DI. Evaluation of near-fatal reactions to allergen immunotherapy injections. J Allergy Clin Immunol 2006;117:169-75. [Crossref] [PubMed]
- Lockey RF, Benedict LM, Turkeltaub PC, et al. Fatalities from immunotherapy (IT) and skin testing (ST). J Allergy Clin Immunol 1987;79:660-77. [Crossref] [PubMed]
- Reid MJ, Lockey RF, Turkeltaub PC, et al. Survey of fatalities from skin testing and immunotherapy 1985-1989. J Allergy Clin Immunol 1993;92:6-15. [Crossref] [PubMed]
- Zeng X, Li J, Xian M, et al. Immediateadverse reactions during subcutaneous standardized house dust mite specific immunotherapy in patients with asthma and/or rhinitis. Chin J Allergy Clin Immunol 2013;7:357-63.
- Liu JL, Ning WX, Li SX, et al. The safety profile of subcutaneous allergen immunotherapy in children with asthma in Hangzhou, East China. Allergol Immunopathol (Madr) 2017;45:541-8. [Crossref] [PubMed]
- Wang CM, Chang CB, Chan MW, et al. Dust mite allergen-specific immunotherapy increases IL4 DNA methylation and induces Der p-specific T cell tolerance in children with allergic asthma. Cell Mol Immunol 2018;15:963-72. [Crossref] [PubMed]
- Luo X, Hong H, Tang J, et al. Increased expression of miR-146a in children with allergic rhinitis after allergen-specific immunotherapy. Allergy Asthma Immunol Res 2016;8:132-40. [Crossref] [PubMed]
- Zeng G, Zheng P, Luo W, et al. Longitudinal profiles of serum specific IgE and IgG4 to Dermatophagoides pteronyssinus allergen and its major components during allergen immunotherapy in a cohort of southern Chinese children. Mol Immunol 2016;74:1-9. [Crossref] [PubMed]


