Thoracoscopic small pulmonary nodule detection using computed tomography-guided cutaneous marking and pleural marking
Introduction
In recent years, small lung nodules have been detected by computed tomography (CT) examination. These nodules are difficult to diagnose by transbronchial biopsy (1). In addition, diagnosis via CT-guided lung biopsy is also difficult owing to breath misalignment. Therefore, the prevalence of surgical resection for pathological diagnosis is increasing (2). Although video-assisted thoracoscopic surgery (VATS) is widely used as a noninvasive surgical approach in wedge resection of small lung tumors (3), it is extremely difficult to identify the position of a small tumor by palpation via a small access port. This is particularly true if the tumor is positioned deep in the lung.
Previously, a CT-guided hook wire has been useful for tumor identification and the localization of small pulmonary nodules. However, if the lung is punctured with the hook wire, this may cause hemoptysis and pneumothorax, and cerebral infarction by air embolism (4,5). In addition, the incidence of pneumothorax is reported to be between 32% and 68%, and the incident of air embolism is 0.62% (6). For that reason, this is considered a dangerous procedure.
The aim of this study was to evaluate our novel method of marking the skin directly above the tumor under CT guidance before surgery with a permanent marker pen, then placing a pleural marker with dye on the parietal pleura (the back side of the skin), followed by performing two-lung ventilation, and marking the visceral pleura just above the tumor by transferring the dye.
Methods
The present study was approved by the Institutional Review Board (IRB) of Kanazawa Medical University Hospital (I265), and the study was registered with UMIN Clinical Trials Registry in Japan (UMIN-CTR ID: UMIN000031848). Individual informed consent was obtained from each patient.
The indications for marking were as follows: a peripheral pulmonary nodule ≤20 mm in diameter, a distance from the nearest pleural surface of ≤20 mm, a planned VATS wedge resection or segmentectomy at Kanazawa Medical University Hospital, and an age of more than 20 years. Patients with no definitive diagnosis before surgery and who needed frozen section diagnosis by VATS wedge resection to perform VATS lobectomy were included. Fifty-four patients were enrolled between February and October 2018.
Technique
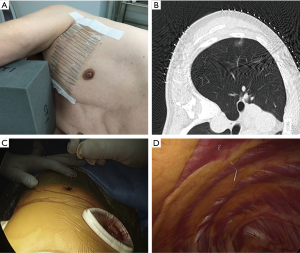
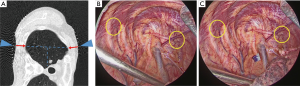
- Prior to surgery, the patients were placed on the CT table in the same lateral position as they would be in during surgery. Guide markers, in the form of cellophane tape and clips, were attached to the body, directly above the site of the possible lung tumor (Figure 1A).
- A CT scan was performed at the time of respiratory arrest and marking was carried out on the skin immediately above the tumor with a permanent marker (Figure 1B).
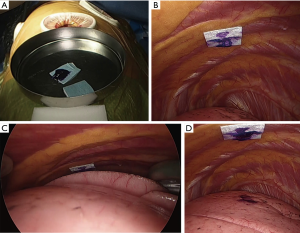
- After the beginning of thoracoscopic surgery, a catheter needle (23G) was inserted into the thoracic cavity from the skin marking site (Figure 1C). After confirming that the tip of the needle was inserted into the parietal pleura (Figure 1D), the pleural marker, which was made using sterilized adhesive tape cut into small 1-cm pieces and mixed with dye (Pyoktanin Blue® 1% water solution; Wako Pure Chemical Industries Ltd., Osaka, Japan) and sterilized gel for ultrasonography (Sterlite Aquasonic 100®; Parker Laboratories Inc., Fairfield, NJ, USA) (Figure 2A), was attached to the same point (Figure 2B).
- After attaching a marker to the parietal pleura, two-lung ventilation was performed at a pressure of 20 cmH2O for 10 seconds to expand the lungs (Figure 2C).
- One-lung ventilation was performed again, and we confirmed that the pigment had adhered to the lung surface. Subsequently, the pleural marker was removed (Figure 2D).
- In order to accurately measure the distance between the marking point and the pleura closest to the tumor, a 3-0 PDS yarn was pierced and ligated at the center of the portion to which the pigment adhered, and finger palpation was performed.
- Partial resection was performed centering on the palpable tumor.
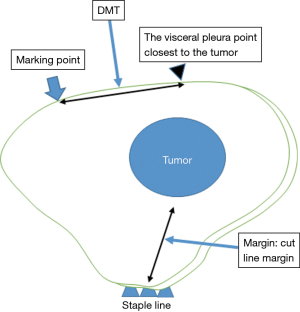
- The presence of tumors was confirmed in all resected lungs by intra-operative frozen section diagnosis. To evaluate the accuracy of the marking before creation of the frozen sections, the distance between the center of the marking and the visceral pleura closest to the tumor (DMT) was measured by pathologists (Figure 3).
- Indirect marking: if the tumor was localized on the back side of a scapula or on the mediastinum side, and pleural marking directly above was difficult, we marked the skin at two points in the vicinity and measured the distance with eye measurement from the puncture needle in the anteroposterior direction (Figure 4A,B,C). We then attached the pleural marker, referring to the distance on the parietal pleura, and marked the visceral pleura directly above the tumor.
In the case of multiple tumors, the evaluation was made only for the largest lesion, and it was a policy to measure only one DMT per case. However, surgeons were allowed to mark multiple tumors, when necessary.
Statistical analysis
We used a t-test to analyze the DMT and other quantitative valuables. The primary endpoint was DMT and the secondary endpoint was the cut line margin (Margin). A statistically significant difference was defined as a value of P<0.05. The EZR software program (Saitama Medical Center, Jichi Medical University, Saitama, Japan) on a personal computer was used for the analysis (7).
In order to verify the changes in surgical outcomes over the period from February to October 2017 with our new marking technique, cases of pulmonary resection with a tumor size less than 20 mm and depth less than 20 mm were extracted (68 cases) as a control group. The control group was compared regarding operation time, the proportion of palpable tumors (palpable rate), and the approach.
Results
In 54 cases enrolled in the present study, three cases were excluded—one due to disappearance of the cutaneous marking, one due to marking near the descending aorta, and one due to avoidance of a partial resection for preoperative diagnosis (Figure 5).
The patient characteristics are listed in Table 1. There were no significant differences between the two groups, except for age and surgical procedure. In the marking group, the mean tumor size was 12.4 mm (vs. 13.1 mm in the control group). Tumor density [consolidation tumor rate (C/T)] was 0.73 (vs. 0.75 in the control group).
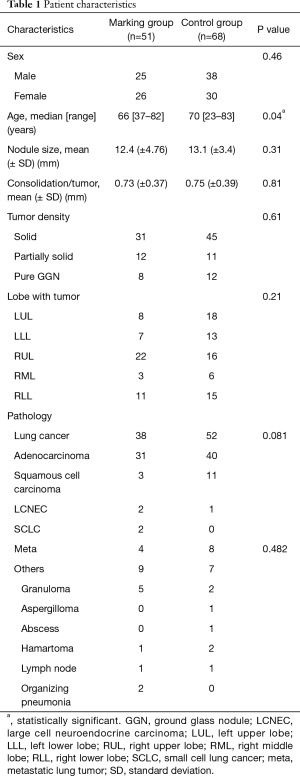
Full table
No morbidity was observed during or after surgery.
The mean DMT was 12.3 mm (0–45 mm) and the mean margin was 13.5 mm (1–45 mm) (Table 2). The mean DMT was 14.2 mm (±10.8 mm) in the indirect marking group and 11.5 mm (±8.8 mm) in the direct marking group. These differences were not significantly different.
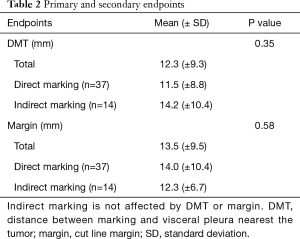
Full table
All tumors were resected completely. In cases where marking defined that the DMT was within 30 mm and the tumor was easily palpable during surgery from the access port, the success rate was 47/51 (92%). In four failure cases, we could not resect the tumors with the marking yarn in two cases, and in the other failure cases, the DMT was not within 30 mm (45 mm in case 4 and 35 mm in case 47).
Surgical procedures in the marking group included 23 wedge resections, 6 segmentectomies, and 22 lobectomies. Two segmentectomies were performed after partial resection, and 20 lobectomies were performed after partial resection. Surgical procedures in the control group included 45 wedge resections, 12 segmentectomies, and 11 lobectomies.
The mean operation time for partial resection was 68.1 minutes in the marking group and 71.7 minutes in the control group. This difference was not significantly different.
The palpable tumor rate was 46/51 in the marking group and 59/68 in the control group (P=0.78). In the marking group, impalpable tumors were resected with wider partial resection in one case, with segmentectomy in one case, and with lobectomy in two cases. There was one case that required conversion to open surgery for palpation of the tumor. However, in the control group, conversion to open surgery was required in nine cases (Table 3).
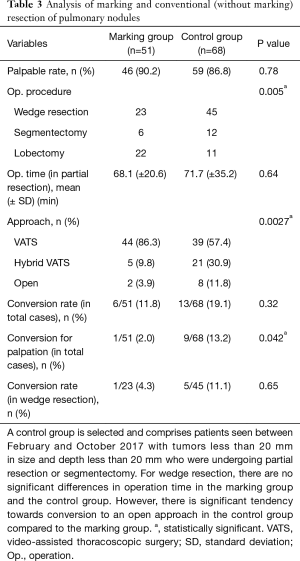
Full table
The surgical approach was VATS in 44 cases in the marking group and 39 cases in the control group. Hybrid VATS was defined as when the skin incision was less than 8 cm, and it was required in five cases in the marking group and 21 cases in the control group. An open approach was defined as when the skin incision was over 8 cm, and it was required in two cases in the marking group and eight cases in the control group. There was a significant difference in approach between the two groups (P=0.0027).
Discussion
Various methods have already been reported for preoperative marking of peripheral pulmonary nodules in thoracoscopic surgery. A procedure using a hook wire that punctures the lung under CT guidance before surgery and involves pigment injection has severe complications, such as cerebral infarction caused by air embolism, as well as pneumothorax or hemothorax (4). Hence, marking of the body surface before surgery under CT guidance without lung puncture has also been reported (8-10).
In Nishida et al.’s method, a puncture needle is inserted transpercutaneously into the extra pleural space, just above the tumor in the CT room before surgery. During surgery, a guide wire is inserted into the pleural cavity. With this guide wire, a cotton ball filled with dye is fixed to the chest wall. It marks the surface of the lung inflated during two-lung ventilation (8). However, this method is very cumbersome as it is necessary to puncture the chest wall in the CT room prior to surgery and local anesthesia is required.
In Kamiyoshihara et al.’s and Mun et al.’s method, CT-guided body surface marking is performed on the skin directly above the tumor with an oil pen before surgery (9,10). During surgery, the needle punctures the skin transpercutaneously using the guide wire, and the cotton ball filled with dye is fixed to the chest wall (9,10).
In these methods, marking the skin before surgery is not very complicated; however, it is somewhat cumbersome to fix the cotton ball to the chest wall. Furthermore, if there is a scapula between the tumor and the skin, it is difficult to attach the pigment to the pleura just above the tumor.
With our method, it is possible to attach the pigment to the visceral pleura just above the tumor, even if the tumor is behind the scapula or in the mediastinal side lung. However, in CT-guided preoperative body surface marking, puncture of the lung is not performed, but there may be some misalignment in the positional relationship between the marking site of the lung surface and the tumor before surgery, so it is necessary to perform intraoperative palpation.
In the present study, the mean DMT was 12 mm. Mun et al. reported a mean distance from the nodule to the marking point of 0.7 cm (range, 0–3 cm). In the present study, there was a case in which the maximum deviation was 4.5 cm, and there were cases where it was impossible to evaluate, such as cases where marking was on the adjacent lung lobe. The cause of discrepancies between the pleural marking site and the location of the tumor was that the marker was dragged and moved by the expanding lungs, and in the case of a puncture in a thick chest wall, the puncture needle was inserted slightly obliquely, as it seemed that position shift would occur. For this reason, in case 3 and beyond, the puncture was performed perpendicularly to the chest wall and the lungs squeezed slightly until just before coming into contact with the chest wall to avoid the pleural marker being shifted.
In cases where the chest wall is thick, the puncture needle is likely to be inserted diagonally, so it is predicted that chest wall thickness affects the DMT. We have examined the distance from the body surface marking portion to the pleura just above the tumor in all cases. Although the correlation between thickness and DMT was examined, no significant association was found. A thick chest wall and obesity were assumed to have little effect on this deviation.
As a localization method for small pulmonary nodules, a method of inserting a dye or a metal marker into the parenchyma around the tumor by a bronchoscope before the operation has been reported (11). The success rate of virtual-assisted lung mapping (VAL-MAP) conducted by Sato et al. was reported to be over 90%, and it is a very useful method for surgery where the impalpable pulmonary nodule needs to be resected. However, it is necessary to prepare a virtual bronchoscopic image before bronchoscopy and to inject the pigment faithfully into the parenchyma whilst referring to this image. This requires advanced technology, which is not available at all facilities.
Because the success rate of marking via this method is 92%, wedge resection with marking of impalpable pulmonary nodules is associated with the risk of tumor resection failure. However, this method can reduce the risk of palpation error. In 2017, before the present study in which we used our marking procedure, we experienced nine cases (13.2%) with conversions caused by impalpable nodules. In the present study, we were able to palpate the nodule via a small access port, except for in one case (1.9%). In other studies, the reported conversion rate for tumor localization has been between 13% and 50%, and some tumor markings are considered useful for reducing the conversion rate (3,12).
In this method, the marking is not complicated and is very safe compared to other localization methods. The pleural marker is made of materials just like the pleural markers used in any operating room. Hence, this procedure can be performed in any facility. Furthermore, it does not need any high technology devices. Though we have not measured the time taken for the marking technique, in partial resection surgery, this method does not show an extension of the operation time compared with that for conventional surgery and it is simple.
We used a sterile echogenic jelly mixed with dye placed on a sterilized surgical tape as a pleural marker, as this prevents the parietal pleura from coming into contact with the echo jelly and dye directly.
A sterilized echo gel is said to be free from tissue degeneration by subcutaneous insertion (13), and is guaranteed to be safe as a material used for marking. In addition to this, with the surgical tape shielding it on the parietal pleura, it is thought to be even safer.The limitation of this technique is that palpation of the tumor remains indispensable at this stage. Position misalignment between the marking and the tumor is on average about 12 mm; however, this is still quite close to the tumor. In four cases (8%), the site of the actual tumor differed greatly from the marking site. Therefore, our technique cannot guarantee that the marking and the tumor will line up perfectly. If we can reduce the likelihood of the pleural marker being dragged, and if the puncture needle can accurately indicate the pleura just above the tumor, it is expected that the marking accuracy will be further improved. Further research for improvements in this method is thus necessary.In conclusion, CT-guided cutaneous marking and pleural marking indicated the location of the tumor with high probability, making it easy to palpate the tumor without a high technology device. This new procedure should be implemented in the clinical setting given its ease of application, safety, and accuracy.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The present study was approved by the Institutional Review Board (IRB) of Kanazawa Medical University Hospital (I265). Individual informed consent was obtained from each patient. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Saghir Z, Dirksen A, Ashraf H, et al. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax 2012;67:296-301. [Crossref] [PubMed]
- Mack MJ, Hazelrigg SR, Landreneau RJ, et al. Thoracoscopy for the diagnosis of the indeterminate solitary pulmonary nodule. Ann Thorac Surg 1993;56:825-30; discussion 830-2. [Crossref] [PubMed]
- Suzuki K, Nagai K, Yoshida J, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest 1999;115:563-8. [Crossref] [PubMed]
- Sortini D, Feo C, Maravegias K, et al. Intrathoracoscopic localization techniques. Review of literature. Surg Endosc 2006;20:1341-7. [Crossref] [PubMed]
- Sakiyama S, Kondo K, Matsuoka H, et al. Fatal air embolism during computed tomography-guided pulmonary marking with a hook-type marker. J Thorac Cardiovasc Surg 2003;126:1207-9. [Crossref] [PubMed]
- Yi JH, Choi PJ, Bang JH, et al. Systemic air embolism after computed tomography-guided hook wire localization: two case reports and literature review. J Thorac Dis 2018;10:E59-64. [Crossref] [PubMed]
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452-8. [Crossref] [PubMed]
- Nishida T, Fujii Y, Akizuki K. Preoperative marking for peripheral pulmonary nodules in thoracoscopic surgery: a new method without piercing the pulmonary parenchyma. Eur J Cardiothorac Surg 2013;44:1131-3. [Crossref] [PubMed]
- Kamiyoshihara M, Ibe T, Kawatani N, et al. A convenient method for identifying a small pulmonary nodule using a dyed swab and geometric mapping. J Thorac Dis 2016;8:2556-61. [Crossref] [PubMed]
- Mun M, Matsuura Y, Nakao M, et al. Noninvasive computed tomography-guided marking technique for peripheral pulmonary nodules. J Thorac Dis 2016;8:S672-76. [Crossref] [PubMed]
- Sato M, Yamada T, Menju T, et al. Virtual-assisted lung mapping: outcome of 100 consecutive cases in a single institute. Eur J Cardiothorac Surg 2015;47:e131-9. [Crossref] [PubMed]
- Cardillo G, Regal M, Sera F, et al. Videothoracoscopic management of the solitary pulmonary nodule: a single-institution study on 429 cases. Ann Thorac Surg 2003;75:1607-11; discussion 1611-2. [Crossref] [PubMed]
- Belavy D, Sunn N, Lau Q, et al. Absence of neurotoxicity with perineural injection of ultrasound gels: assessment using an animal model. BMC Anesthesiol 2013;13:18. [Crossref] [PubMed]