Transbronchial lung biopsy and pneumothorax
Introduction
Bronchoscope is needed in every transbronchial operation and bronchoscopists usually have such a concept: diagnostic specimens must be taken even with some risks. It has several meanings: (I) taking risks are inevitable and valuable; (II) these risks must have ways to solve and they are best to be predictable; (III) to prevent and solve these risks are based on fully preparation, skillful operation, rich experience and confidence) (1-5).
Transbronchial biopsy is the most commonly used in bronchoscope operation and it wildly used in lung diseases diagnosis. With the development of technology and improvement of equipments, it will be used more wildly, more accurate and safer. With bronchoscope, more other operations can be done. By the present transbronchial operations may be divided into four kinds: (I) the operations in the larger airway: therapeutic operations almost all belong to this category, such as microwave, laser, argon, high frequency electric coagulation/cutting, cryotherapy, thermo forming, balloon expansion, stent implantation, intracavitary brachytherapy, etc. Absolutely regular tissue and mucosa biopsy are also included; (II) the operations outside the larger airway lumen: bronchial needle aspiration mainly was carried out according to Wang’s localization methods, but now usually with endobronchial ultrasonic (EBUS) guidance; (III) guided operations in the peripheral airway: may be guided by X-ray fluoroscopy, CT (even CT fluoroscopy), endoscopic ultrasound (EUS), electromagnetic navigation. New guided-bronchoscopy technologies have been developed to improve the yield of transbronchial biopsy for peripheral nodes diagnosis: virtual bronchoscopy (VB), radial EBUS (R-EBUS), ultrathin bronchoscope, and guide sheath; (IV) non-guide operations in the peripheral airway: traditional transbronchial lung biopsy (5-20).
Endobronchial interventional operations
Endobronchial interventional operations will lead the common following complications: anesthetic accident, discomfort from pre-operation medication, hemorrhage, hypoxia, laryngeal edema, bronchospasm, aspiration, infection, cardiovascular events, etc. Pneumothorax and pneumomediastinum are also included. Intraoperative or postoperative dyspnea must be paid attention, especially after the spasmolysis. Pneumothorax can be confirmed by careful physical examination and chest X-ray. There was delayed pneumothorax, such as a case in 5 days after operation. With small size of pneumothorax and inconspicuous dyspnea, the patients may be taken oxygen therapy and close observation. With large size of pneumothorax or pneumomediastinum, patients should need more active treatment, including chest tube drainage.
The operations in the larger airways include the therapeutic procedures and conventional tissue and mucosal biopsy, scraping or brush. Because of the restriction of the outer diameter of bronchoscope, most operations only are carried out in the proximal lumen of level 9 bronchi. The incidence rate of iatrogenic pneumothorax was less than 1% in most surveys because the operations are far away from visceral pleura. Penetrable trauma in the tracheal or bronchial wall might result in the pneumothorax, even pneumomediastinum (20-30).
New treatment procedures
Some new treatment procedures may change the structure or air distribution of lung, such as bronchoscopic lung volume reduction for some end-stage COPD. The placement of one-way endobronchial valve may lead to the collapse of corresponding lobe or segment which has already lost its function. But in the same lung, especially in the same lobe, more air would be inhaled into other segments promptly, which may lead to pneumothorax. It has already been reported about these death cases.
Transbronchial needle aspiration for cytologic or histologic specimens is the most important method of diagnosis of the lesions outside larger airway lumen. Except in the upper trachea, pneumothorax and pneumomediastinum have a very low incidence rate (no less than 1%) and is not relative to any puncture maneuver.
The guided operation in peripheral airway are mainly used for peripheral diffuse lesions or occupying lesions. Making sure the appliances such as forceps or needle will not touch the visceral pleura, most operations will not lead to pneumothorax. And they will be not also easy to result in pneumomediastinum because of the distance to mediastinum. There may be not different incidence rate of pneumothorax resulted from the same operations on different lobe or segment. While operating under X-ray fluoroscopic guidance, the risk will increase relatively. Biopsy forceps need to be controlled carefully to avoid touching to the pleura. In Figure 1A, the forcep seemed not to touch the pleura in X-ray, but it really reached the dangerous region in CT scanning (Figure 1B). During the operations, forceps would be opened and moved forward appreciably after to reach the lesion, then be closed for the biopsy at the end of expiration (30-35).
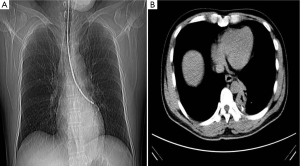
The incidence rate of pneumothorax resulted from the guided operations in peripheral airway is generally less than 1-2%, even with larger cup forceps, crocodile jaw forceps, cryoprobes or endobronchial electrocautery.
Linden and his colleagues reported a higher incidence rate of pneumothorax, 5-8% with electromagnetic navigation bronchoscopy (ENB)-directed biopsies and guide the placement of fiducial markers for stereotactic radiosurgery. But they also emphasized that the patients with underlying lung disease such as COPD were prone to happen. Such high incidence rate was also reported in minority surveys on EBUS or EBUS + ENB.
In general, peripheral lesions may be diagnosed by bronchoscopy with biopsy but brush, scraping or lavage. Needle aspiration is not used commonly which have few reports about it. In the detail of maneuver, put the needle at a right place and then send it directly into the lesions. If pierce direction deviation, withdraw slightly and rotate gently, then move it forward again. Furthermore operators may use cough maneuver or send the needle forward during the inhalation and pierce passively the right region during the expiration (25,30-40).
Non-guided operation
Non-guided operation in the peripheral airway will decrease the positive rate obviously and have a greater risk than X-ray fluoroscopy guided operation, in other words it is the most dangerous operation. The operations mainly are used for diagnosis of lesions involving most area of at least one lung segment. If it is a diffuse lesion, operators usually choose to biopsy on dorsal, outer or posterior segment of the lower lobe (B6, B9, B10). Due to no guidance, operators are often relatively conservative who may not biopsy on the dangerous regions such as apical segment. So there is a very different of the reported incidence rate of pneumothorax. Operators may control the depth of forcep through the sense of touch or patients’ chest pain reaction in order to prevent relatively the occurrence of pneumothorax. Most surveys show that the incidence rate of pneumothorax resulted from such operations is only at about 0.2-5.5%, higher on the patients with underlying lung disease such as COPD. Once with pneumothorax, half of patients require chest tube placement (37-39). But it is not reported the death cases caused by the pneumothorax. There are some the detail of maneuvers in the following:
- Put bronchoscope to right segment (or subsegment);
- Put forceps slowly into the target region until it meets the resistance or patients feel mild chest pain, then withdraw the forceps 1-2 cm;
- Patients inhale deeply. At the end of deep inspiration, assistant opens forceps and move forward until meet the resistance (usually about 1 cm);
- Patients expirate deeply. At the end of deep expiration, assistant closes forceps for the biopsy;
- If forceps can not reach the predetermined depth which meet obvious resistance, it may meet with small bronchus bifurcate cristae. Slightly withdraw forceps and gently rotate, then move it forward again.
In order to improve the positive rate, 3-4 pieces of tissues would be taken in different pulmonary segment or subsegment with forceps, but it will increase the incidence rate of pneumothorax. Meanwhile biopsy with serrated edge forceps can raise relatively the positive rate, and increase simultaneously the chance of bleeding and pneumothorax (20-28).
Safety of transbronchial lung biopsy
The safety of transbronchial lung biopsy is few reported on several special patients such as ventilating patients. O’Brien and his colleagues were carried out transbronchial lung biopsy for 83 times on 71 ventilating patients. And the results showed that the incidence of pneumothorax was the highest among all complications, ten cases (14.3%). But the pneumothorax was comparatively mild which does not need to chest tube drainage and nobody died. A total of 29 cases (34.9%) got the histologic diagnosis and correct their treatment. So transbronchial lung biopsy is a relatively safe and valuable. Others reported broadly similar to mild pneumothorax, the incidence of not more than 28% (1-5).
Some researchers believe that the proficiency on the skills had no significant effect on the incidence of pneumothorax. But Stather and his colleagues showed that trainee participation in advanced diagnostic bronchoscopy utilizing the apprenticeship model increased the time of procedure, increased the amount of sedation used and resulted in a trend to increased complications. Although no difference in total incidence rate of complications, but pneumothorax was an exception. Pneumothorax incidence in group without trainee was zero, but the rate in trainee group was 11/346 (2.2%), of which 7 patients required chest tube drainage. Among all the operations, the incidence of pneumothorax resulted from endobronchial ultrasound (EBUS) guided transbronchial needle aspiration and lung biopsy was the most, occuring in 5/30 (16.7%) (10-18).
Summary
In order to reduce the complications such as pneumothorax, every operator should know more about anatomic structure, characteristics in the specific situation, skills of selected tools or methods. While choosing the right patients with suitable instruments at the right place and time for proper operation, the operators would really improve the positive rate and security.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Asano F, Aoe M, Ohsaki Y, et al. Deaths and complications associated with respiratory endoscopy: a survey by the Japan Society for Respiratory Endoscopy in 2010. Respirology 2012;17:478-85. [PubMed]
- Bulpa PA, Dive AM, Mertens L, et al. Combined bronchoalveolar lavage and transbronchial lung biopsy: safety and yield in ventilated patients. Eur Respir J 2003;21:489-94. [PubMed]
- Du Rand IA, Barber PV, Goldring J, et al. British Thoracic Society guideline for advanced diagnostic and therapeutic flexible bronchoscopy in adults. Thorax 2011;66 Suppl 3:iii1-21. [PubMed]
- Eapen GA, Shah AM, Lei X, et al. Complications, consequences, and practice patterns of endobronchial ultrasound-guided transbronchial needle aspiration: Results of the AQuIRE registry. Chest 2013;143:1044-53. [PubMed]
- Eberhardt R, Anantham D, Ernst A, et al. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am J Respir Crit Care Med 2007;176:36-41. [PubMed]
- Ghofrani M, Kim B. Diagnosis of pneumothorax on F-18 FDG PET after transbronchial biopsy. Clin Nucl Med 2005;30:692-4. [PubMed]
- Huang CT, Ruan SY, Liao WY, et al. Risk factors of pneumothorax after endobronchial ultrasound-guided transbronchial biopsy for peripheral lung lesions. PLoS One 2012;7:e49125. [PubMed]
- Linden PA. Use of navigation bronchoscopy for biopsy and endobronchial fiducial placement. Innovations (Phila) 2011;6:271-5. [PubMed]
- O’Brien JD, Ettinger NA, Shevlin D, et al. Safety and yield of transbronchial biopsy in mechanically ventilated patients. Crit Care Med 1997;25:440-6. [PubMed]
- Shulimzon TR, Israel Lung Association Task Force. Flexible bronchoscopy in Israel 2010: evidence-based clinical practice guidelines for the adult patient. A concise summary of the recommendations of the Israel Lung Association Task Force. Isr Med Assoc J 2010;12:69-73. [PubMed]
- Stather DR, Maceachern P, Chee A, et al. Trainee impact on advanced diagnostic bronchoscopy: an analysis of 607 consecutive procedures in an interventional pulmonary practice. Respirology 2013;18:179-84. [PubMed]
- Tukey MH, Wiener RS. Population-based estimates of transbronchial lung biopsy utilization and complications. Respir Med 2012;106:1559-65. [PubMed]
- Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012;142:385-93. [PubMed]
- Zhang J. Transbronchial biopsy. In: Li Q. eds. Respiratory endoscopics. Shanghai: Shanghai Science and Technology Press, 2003:103-11.
- Tsakiridis K, Mpakas A, Kesisis G, et al. Lung inflammatory response syndrome after cardiac-operations and treatment of lornoxicam. J Thorac Dis 2014;6 Suppl 1:S78-98. [PubMed]
- Tsakiridis K, Zarogoulidis P, Vretzkakis G, et al. Effect of lornoxicam in lung inflammatory response syndrome after operations for cardiac surgery with cardiopulmonary bypass. J Thorac Dis 2014;6 Suppl 1:S7-20. [PubMed]
- Argiriou M, Kolokotron SM, Sakellaridis T, et al. Right heart failure post left ventricular assist device implantation. J Thorac Dis 2014;6 Suppl 1:S52-9. [PubMed]
- Madesis A, Tsakiridis K, Zarogoulidis P, et al. Review of mitral valve insufficiency: repair or replacement. J Thorac Dis 2014;6:S39-51. [PubMed]
- Siminelakis S, Kakourou A, Batistatou A, et al. Thirteen years follow-up of heart myxoma operated patients: what is the appropriate surgical technique? J Thorac Dis 2014;6 Suppl 1:S32-8. [PubMed]
- Foroulis CN, Kleontas A, Karatzopoulos A, et al. Early reoperation performed for the management of complications in patients undergoing general thoracic surgical procedures. J Thorac Dis 2014;6 Suppl 1:S21-31. [PubMed]
- Nikolaos P, Vasilios L, Efstratios K, et al. Therapeutic modalities for Pancoast tumors. J Thorac Dis 2014;6 Suppl 1:S180-93. [PubMed]
- Koutentakis M, Siminelakis S, Korantzopoulos P, et al. Surgical management of cardiac implantable electronic device infections. J Thorac Dis 2014;6 Suppl 1:S173-9. [PubMed]
- Spyratos D, Zarogoulidis P, Porpodis K, et al. Preoperative evaluation for lung cancer resection. J Thorac Dis 2014;6 Suppl 1:S162-6. [PubMed]
- Panagopoulos N, Leivaditis V, Koletsis E, et al. Pancoast tumors: characteristics and preoperative assessment. J Thorac Dis 2014;6 Suppl 1:S108-15. [PubMed]
- Visouli AN, Darwiche K, Mpakas A, et al. Catamenial pneumothorax: a rare entity? Report of 5 cases and review of the literature. J Thorac Dis 2012;4 Suppl 1:17-31. [PubMed]
- Zarogoulidis P, Chatzaki E, Hohenforst-Schmidt W, et al. Management of malignant pleural effusion by suicide gene therapy in advanced stage lung cancer: a case series and literature review. Cancer Gene Ther 2012;19:593-600. [PubMed]
- Papaioannou M, Pitsiou G, Manika K, et al. COPD Assessment Test: A Simple Tool to Evaluate Disease Severity and Response to Treatment. COPD 2014;11:489-95. [PubMed]
- Porpodis K, Zarogoulidis P, Spyratos D, et al. Pneumothorax and asthma. J Thorac Dis 2014;6 Suppl 1:S152-61. [PubMed]
- Papaiwannou A, Zarogoulidis P, Porpodis K, et al. Asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): current literature review. J Thorac Dis 2014;6 Suppl 1:S146-51. [PubMed]
- Zarogoulidis P, Porpodis K, Kioumis I, et al. Experimentation with inhaled bronchodilators and corticosteroids. Int J Pharm 2014;461:411-8. [PubMed]
- Bai C, Huang H, Yao X, et al. Application of flexible bronchoscopy in inhalation lung injury. Diagn Pathol 2013;8:174. [PubMed]
- Zarogoulidis P, Kioumis I, Porpodis K, et al. Clinical experimentation with aerosol antibiotics: current and future methods of administration. Drug Des Devel Ther 2013;7:1115-34. [PubMed]
- Zarogoulidis P, Pataka A, Terzi E, et al. Intensive care unit and lung cancer: when should we intubate? J Thorac Dis 2013;5 Suppl 4:S407-12. [PubMed]
- Hohenforst-Schmidt W, Petermann A, Visouli A, et al. Successful application of extracorporeal membrane oxygenation due to pulmonary hemorrhage secondary to granulomatosis with polyangiitis. Drug Des Devel Ther 2013;7:627-33. [PubMed]
- Zarogoulidis P, Kontakiotis T, Tsakiridis K, et al. Difficult airway and difficult intubation in postintubation tracheal stenosis: a case report and literature review. Ther Clin Risk Manag 2012;8:279-86. [PubMed]
- Boskovic T, Stanic J, Pena-Karan S, et al. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J Thorac Dis 2014;6 Suppl 1:S99-107. [PubMed]
- Hohenforst-Schmidt W, Zarogoulidis P, Vogl T, et al. Cone Beam Computertomography (CBCT) in Interventional Chest Medicine - High Feasibility for Endobronchial Realtime Navigation. J Cancer 2014;5:231-41. [PubMed]
- Hohenforst-Schmidt W, Banckwitz R, Zarogoulidis P, et al. Radiation Exposure of Patients by Cone Beam CT during Endobronchial Navigation - A Phantom Study. J Cancer 2014;5:192-202. [PubMed]
- Tsakiridis K, Zarogoulidis P. An interview between a pulmonologist and a thoracic surgeon-Pleuroscopy: the reappearance of an old definition. J Thorac Dis 2013;5 Suppl 4:S449-51. [PubMed]
- Yarmus L, Akulian J, Gilbert C, et al. Optimizing endobronchial ultrasound for molecular analysis. How many passes are needed? Ann Am Thorac Soc 2013;10:636-43. [PubMed]


