Retrospective study of efficacy of adjuvant chemotherapy using tegafur-uracil in patients with non-small cell lung cancer with primary tumor size of 4.1–5.0 cm
Introduction
In the previous 7th edition of the TNM staging system for lung cancer issued by the International Union Against Cancer (UICC-7) (1), the T stage of tumors with 3.1–5.0 cm in maximum tumor size was defined as T2a, and patients with T2aN0M0 disease were classified into Stage IB. In the guideline issued by The Japan Lung Cancer Society (JLCS) (2) conformable to UICC-7, administration of adjuvant chemotherapy using oral intake of tegafur-uracil (UFT) has been recommended for the patients with pathological stage IB non-small cell lung cancer (NSCLC) and administration of adjuvant chemotherapy using cisplatin doublet has been recommended for patients with p-stage IIA or more progressive stage NSCLC. But, in the latest 8th edition of the TNM staging system for lung cancer issued by the International Union Against Cancer (UICC-8) (3), T stage of tumors with 3.1–5.0 cm in size has been subdivided and tumors with 4.1–5.0 cm in size has been up-graded to T2b, and consequently, patients with T2bN0M0 disease has been up-staged to stage IIA reflecting their worth prognosis (4). However, Japanese guideline has not yet corresponded to this revision, it is still controversial that patients with p-stage IIA disease according to UICC-8 should receive adjuvant UFT therapy the same as before. In this study, we retrospectively evaluated the efficacy of adjuvant UFT therapy for patients with NSCLC having their tumor size of 4.1–5.0 cm.
Methods
A total of 981 consecutive patients with NSCLC who underwent pulmonary resection at 9 hospitals affiliated with Yokohama City University from January 2005 through December 2007 were evaluated. Among these patients, 130 patients who underwent complete anatomic resection (lobectomy or greater with systematic lymph-node dissection) without receiving induction chemotherapy or radiotherapy and pathologically diagnosed having p-Stage IB disease according to UICC-7 with primary tumor size ≥3.1 cm were enrolled in this study. After receiving approval from the institutional review boards of the participating hospitals, we retrospectively reviewed the medical records of each patient and recorded the clinicopathological variables. As for adjuvant chemotherapy, oral intake of 250 mg/m2/day of UFT for 2 years was basically introduced, but dose reduction or discontinuation, change to other regimen were allowed by the decision of attending physicians. Postoperative follow-up examinations were done by the authors and their colleagues by every 3–6 months at each hospital for at least 5 years after resection. Overall survival (OS) was defined as the period between the date of surgical resection and the date of death from any cause. Clinicopathological variables were contrasted between groups with the use of the Mann-Whitney U test and χ2 test. Survival curves were estimated using the Kaplan-Meier method and contrasted between groups with the log-rank test, and hazard ratio (HR) and 95% confidence interval (CI) were calculated using a Cox proportional-hazards model. All analyses were performed using SPSS Statistics 24 (IBM Corp., New York, NY, USA), with P less than 0.05 considered to indicate significance.
Results
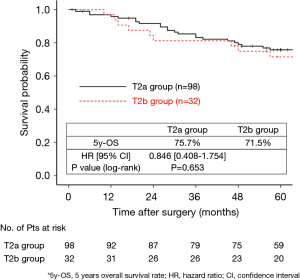
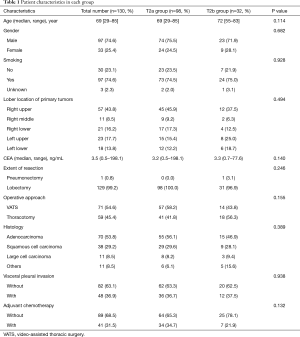
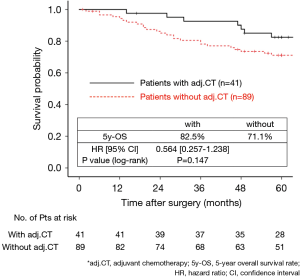
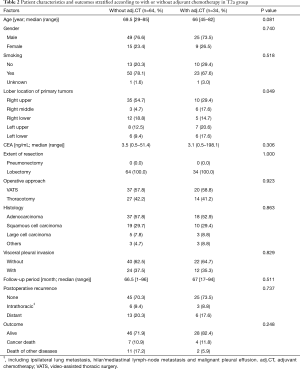
The 130 subjects comprised 97 men and 33 women with a mean age of 69 years (range, 29–85). Ninety-eight patients had their primary tumor of 3.1–4.0 cm in maximum diameter (T2a group), while 32 patients had their primary tumor of 4.1–5.0 cm in maximum diameter (T2b group). About the histology, more than half of patients had adenocarcinoma, while about 30% of patients had squamous cell carcinoma. Adjuvant chemotherapy was administrated only in 41 patients (31.5%). The patients’ characteristics are shown according to the tumor size in Table 1. There was no significant difference in any patients’ characteristics between T2a group and T2b group. The median follow-up after surgery was 67.0 months in whole (range, 1–96) and the 5-year OS rate of the 130 subjects was 74.7%. Patients in T2a group tended to have better outcome than did patients in T2b group, but not statistically significant (Figure 1). Postoperative OS curves stratified according to with or without adjuvant chemotherapy are shown in Figure 2. Patients with adjuvant chemotherapy showed better outcome than did patients without adjuvant chemotherapy, but not statistically significant (HR 0.564; 95% CI, 0.257–1.238, P=0.147). On subgroup analyses among T2a group and T2b group, there was no significant difference in any patients’ characteristics between patients with adjuvant chemotherapy and patients without adjuvant chemotherapy in each subgroup (Tables 2,3). Postoperative OS curves stratified according to with or without adjuvant chemotherapy in T2a group and T2b group are shown in Figures 3,4. In T2a group, patients with adjuvant chemotherapy had tendency toward have better outcome than did patients without adjuvant chemotherapy (HR 0.504; 95% CI, 0.202–1.255, P=0.132). Also, similar tendency had been shown even in T2b group (HR 0.855; 95% CI, 0.181–4.033, P=0.843), but there was not statistical difference between patients with adjuvant chemotherapy and patients without adjuvant chemotherapy in both subgroups.
Full table
Full table
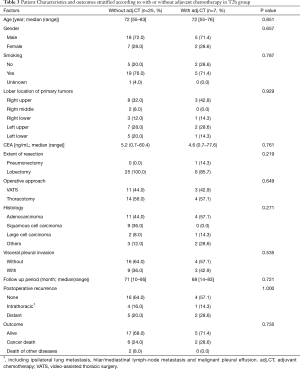
Full table
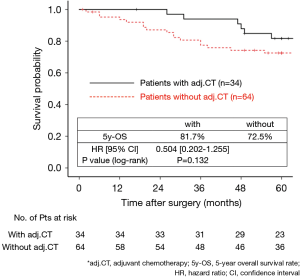
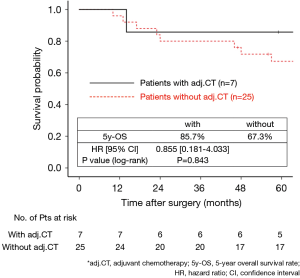
Discussion
In the UICC-7, which was the world’s first unified TNM staging system revised in 2010, the T stage of tumors with 3.1–5.0 cm in maximum tumor size was defined as T2a, and patients with T2aN0M0 disease were classified into Stage IB. Based on the UICC-7, JLCS published the Japanese Clinical Guidelines for the Management of Lung Cancer in 2014 and revised in 2016 (2). In this guideline, they have recommended adjuvant chemotherapy using UFT for patients with completely resected p-stage IB NSCLC to improve their postoperative outcome. UFT is an oral fluorouracil preparation that combines tegafur, a prodrug of 5-fluorouracil, with uracil, which inhibits dihydropyrimidine dehydrogenase, the rate-limiting enzyme responsible for 5-fluorouracil catabolism (5). In Japan, UFT is widely used for postoperative adjuvant chemotherapy, and recommendation for adjuvant chemotherapy for completely resected NSCLC patients has been based on the results of Kato et al. (6) and Hamada et al. (5). Kato et al. conducted phase III prospective randomized controlled trial (RCT) among patients with completely resected lung adenocarcinomas which had no invasion to surrounding tissue and no lymphatic/distant metastases and showed better prognosis in patients with adjuvant UFT therapy than patients without adjuvant UFT therapy. Especially in patients with tumors larger than or equal to 3.1 cm, adjuvant UFT therapy gave large benefit with improve of their 5-year OS by 11% (5-year OS 85% in patients with UFT and 74% in patients without UFT) in their study. Subsequently, Hamada et al. evaluated the efficacy of adjuvant UFT therapy by meta-analysis of 6 CRT including Kato et al. and showed that adjuvant UFT improve postoperative survival of patients with completely resected lung adenocarcinomas which had no invasion to surrounding tissue and no lymphatic/distant metastases, and that results have led to the recommendation of JLCS. However, in the latest UICC-8 which was revised in 2016, T stage of tumors with 3.1–5.0 cm in size has been subdivided and tumors with 4.1–5.0 cm in size has been up-graded to T2b, and consequently, patients with T2bN0M0 disease has been up-staged to stage IIA reflecting the results of analyses among 77,156 lung cancer patients conducted by The International Association for the Study of Lung Cancer (4). But there were no subgroup analyses classified by larger or less than 4.0 cm in tumor sizes in the study of Kato et al. and Hamada et al., it is still controversial whether patients with completely resected p-stage IIA NSCLC according to UICC-8 should receive adjuvant UFT therapy the same as patients with p-stage IB according to UICC-7 or receive adjuvant Cisplatin doublet therapy as new p-stage IIA according to UICC-8.
In our study, although there was no statistical significance, patients with adjuvant UFT therapy showed better postoperative survival than did patients without adjuvant UFT in T2a group, and this result support the recommendation of JLCS. Moreover, in T2b group, patients with adjuvant UFT also tended to show tendency toward show better postoperative survival than did patients without adjuvant UFT. According to our results, although we could not show statistically significant differences, we consider that adjuvant UFT therapy has potential to improve postoperative survival even in patients with p-stage IIA NSCLC according to UICC-8. Recently, Tsuboi et al. re-evaluated the data used in the study of Kato et al. and found it to conform to the rule of UICC-8 and reported that adjuvant UFT therapy improved postoperative survival in patients with p-T2bN0M0 stage IIA NSCLC according to UICC-8 (7). It supports our result, but because this study had only 50 subjects in T2b group, they could not show statistically significant difference (HR 0.55; 95% CI, 0.10–3.07) as same as our study.
On the other hand, it is difficult to presume the efficacy of adjuvant chemotherapy in patients with p-stage IIA NSCLC according to UICC-8 from the trend of Western countries, because there was no recommendation to introduce adjuvant chemotherapy to patients with p-stage IB according to UICC-7 (8,9), which based on the result of LACE study (10). Park et al. (11) and Hung et al. (12) reported the efficacy of adjuvant chemotherapy for patients with p-stage IB NSCLC diagnosed according to UICC-7, but there were not subgroup analyses classified according to tumor size larger or less than 4.0 cm. Only in subgroup analysis of CALGB9633 study, which was conducted by Strauss et al. to evaluate the efficacy of adjuvant chemotherapy using Carboplatin + Paclitaxel (CBDCA + PTX) among the patients with tumors larger than or equal to 3.1 cm and no lymphatic/distant metastases, adjuvant CBDCA + PTX therapy showed improvement of postoperative survival in patients with tumor more than or equal to 4.1 cm subgroup (13). Considering their results, adjuvant chemotherapy using platinum-base doublet including CBDCA + PTX may more suitable for patients with p-stage IIA NSCLC according to UICC-8. However, CALGB9633 study could not show statistical significance in primary endpoint due to the lack of statistical power, and it meant that there was also no statistical significance in their subgroup analysis. Thus, any previous studies including ours could not have clarified the optimal regimen of adjuvant chemotherapy and even its efficacy for patients with p-stage IIA NSCLC according to UICC-8. In addition, there is important difference between UICC-7 and UICC-8 in the rule of T staging. UICC-7 and further previous edition of TNM staging system had used the “total size of tumor” to decide the T staging, whereas UICC-8 has used the “invasive size of tumor”. Due to this important difference between previous edition of TNM staging system and newest UICC-8, it is uncertain that the results of studies using previous TNM staging system could directly adopt to the UICC-8. So, we consider that further large cohort study using the rule of T staging according UICC-8 is necessary to evaluate the efficacy and optimal regimen of adjuvant chemotherapy for patients with p-stage IIA NSCLC according to UICC-8.
Our study was retrospective and thus had several important limitations. A major limitation is the small sample size, especially in T2b group patients. Although our study could not show statistical difference in survival between patients with adjuvant chemotherapy and patients without even in whole, we consider that it is because the lack of statistical power due to the small sample size. Secondly, we had no important data about adjuvant chemotherapy such as the percentage of patients who changed regimen from UFT to others, percentage of patients who complete planed adjuvant therapy, occurring rate and grade of adverse events of adjuvant chemotherapy, and so on. Moreover, we were not certain the reason why about 70% of patients in whole did not introduced adjuvant chemotherapy. Because we used “Total size of tumor” to classify patients into both group due to the lack of data about “Invasive size of tumor”, our patients in T2a group and T2b group are not strictly equivalent to T2a and T2b in UICC-8. We consider that such patients who had tumor with more than 4.0 cm in total size and less than 4.0 cm in invasive size are very rare and our classification of T2a group and T2b group were almost equivalent to the classification in UISS-8, but this became third limitation. Nevertheless, our study could not show statistical significance and had some limitation, we consider that adjuvant UFT therapy might have potential to improve postoperative survival in patients with p-stage IIA NSCLC according to UICC-8. We hope this study give rise to further studies about adjuvant chemotherapy for these patients.
Conclusions
Although we could not show the statistical effectiveness because of the lack of statistical power, we consider that adjuvant chemotherapy using UFT has potential to improve postoperative survival of patients with completely resected p-stage IIA NSCLC according to UICC-8. Further large cohort study using the rule of T staging according UICC-8 is necessary to evaluate the efficacy and explore the optimal regimen of adjuvant chemotherapy for these patients.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional ethics board of Yokohama City University Medical Center (No. B170200060). The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumors. 7th edition. Oxford: Wiley-Blackwell, 2009:136-7.
- The Japan Lung Cancer Society. Adjuvant chemotherapy for non-small cell lung cancer; pathorogical stage I. Available online: https://www.haigan.gr.jp/modules/guideline/index.php?content_id=32
- Brierly JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumors. 8th edition. Oxford: Wiley-Blackwell, 2017:114-20.
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003.
- Hamada C, Tanaka F, Ohta M, et al. Meta-analysis of postoperative adjuvant chemotherapy with tegafur-uracil in non-small-cell lung cancer. J Clin Oncol 2005;23:4999-5006. [Crossref] [PubMed]
- Kato H, Ichinose Y, Ohta M, et al. A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med 2004;350:1713-21. [Crossref] [PubMed]
- Tsuboi M, Hamada C, Kato H, et al. The Effect of Tegafur-Uracil on Survival in T Categories as Defined in the Eighth Edition of the TNM Classification: An Exploratory Analysis of Postoperative Adjuvant Tegafur-Uracil on Survival in Patients with Adenocarcinoma of the Lung. Chemotherapy 2017;62:357-60.
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313S.
- Kris MG, Gaspar LE, Chaft JE, et al. Adjuvant Systemic Therapy and Adjuvant Radiation Therapy for Stage I to IIIA Completely Resected Non-Small-Cell Lung Cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline Update. J Clin Oncol 2017;35:2960-74. [Crossref] [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- Park SY, Lee JG, Kim J, et al. Efficacy of platinum-based adjuvant chemotherapy in T2aN0 stage IB non-small cell lung cancer. J Cardiothorac Surg 2013;8:151. [Crossref] [PubMed]
- Hung JJ, Wu YC, Chou TY, et al. Adjuvant Chemotherapy Improves the Probability of Freedom From Recurrence in Patients With Resected Stage IB Lung Adenocarcinoma. Ann Thorac Surg 2016;101:1346-53. [Crossref] [PubMed]
- Strauss GM, Herndon JE 2nd, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 2008;26:5043-51. [Crossref] [PubMed]