Pulmonary echinococcosis in China
Pulmonary echinococcosis, also known as lung hydatid disease or hydatidosis, is a zoonotic parasitic disease caused by the larval stage of cestodes belonging to the genus Echinococcus (1).
Etiology and route of transmission
There are two pathogenic species of Echinococcus (E. granulosus and E. multilocularis) in China, with E. granulosus being the most prevalent (accounting for about 90% of cases). The life cycle of the Echinococcus spp. requires two mammalian hosts for its completion. These worms have three stages in their life cycle: egg, larva, and adult.
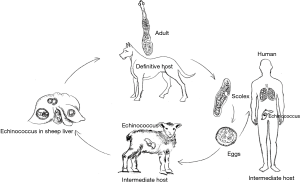
Adult worms live in the small intestines of canines (e.g., dogs, wolves, wild dogs, jackals, and foxes) and felines, and the gravid proglottids or eggs are excreted via the feces. The eggs of E. granulosus are developed into echinococcus after being ingested by intermediate hosts (usually hoofed animals including sheep, cattle, pigs, horses, and camels), whereas the eggs of E. multilocularis hatch in the intestine of rodents and Lagomorpha (e.g., pikas and Qinghai voles). The eggs come out with the feces and contaminate water, soil, pasture land, livestock farms, vegetables, or dog fur. In pastoral areas, people have close relationships with dogs, the main definitive hosts. These eggs can contaminate people’s hands, especially children’s hands, through the dog’s fur. In addition, poor sanitary conditions and hygienic habits eventually lead to food contamination. After eggs are ingested from contaminated water sources or food by mistake, the oncospheres (embryos) are released in the duodenum under the action of digestive juice and then enter the intestinal capillaries and venules before they flow into the liver through the portal vein system. Most embryos lodge in the liver, forming hepatic hydatid cysts. Some of the embryos in the liver can enter pulmonary circulation through the inferior vena cava, forming pulmonary hydatid cysts. Some embryos pass directly through the mesenteric lymphatic system and then the thoracic duct to reach the lungs without passing through the liver. It has also been reported that the eggs in dry and windy areas can float with the wind and develop into pulmonary hydatid cysts directly after being inhaled. In rare cases, the eggs can invade other organs and tissues through pulmonary circulation, and then grow into cysts in the brain, bone, or eyes. In humans, hepatic hydatid cysts are the most common, accounting for 65% to 75%, of cases, followed by pulmonary hydatid cysts, accounting for 15% to 30% of cases. After the canines have eaten the hydatid cyst-containing viscera from livestock, they become infected again, and the cycle restarts (Figure 1).
Pathology
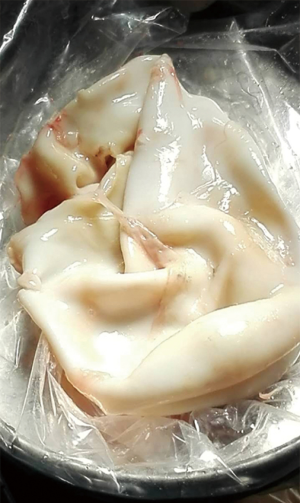
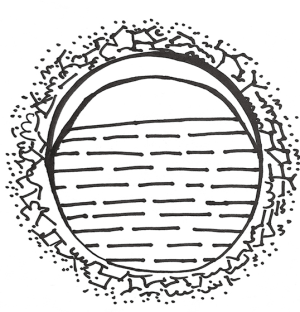
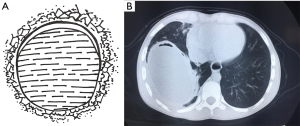
A pulmonary hydatid cyst is composed of a pericyst and endocyst, with a potential gap between them. The gap is loose in the absence of infection. The pericyst is actually a fibrous envelope formed after the lung tissue is compressed, and its surrounding lung tissue often has inflammatory reactions such as congestion and edema. The endocyst is the proper wall of the hydatid cyst and can be divided into two layers: the ectocyst, which is a laminated membrane that is white, powder-like, fragile, and easy to rupture; it can protect the germinal layer cells and absorb nutrients. The innermost layer is the germinal layer closely attached to the laminated membrane; it consists of a row of cells and has a strong reproductive capacity; also, it can produce brood capsules, protoscoleces, and cystic fluid. Many antigens exist in the cystic fluid, which also contains daughter cysts and scolices. Once the cyst ruptures, cystic fluid leaks into the pleural cavity and induces varying degrees of allergic reactions, with the severe cases even leading to anaphylactic shock or death. A large number of scolices can overflow with cystic fluid, forming secondary pleural hydatid cysts (Figure 2).
Epidemiology
Cystic echinococcosis has a worldwide distribution and is more prevalent in countries and areas with developed animal husbandry. Considerable echinococcosis-related public health problems are endemic to areas such as the Mediterranean region, Australia, New Zealand, the Middle East, Alaska (USA), Canada, South America and the indigenous tribes of the United States.
China is one of the countries reporting the highest prevalence of human echinococcosis in the world. According to China’s National Survey on Major Human Parasitic Diseases in 2004, the human prevalence rate of echinococcosis in epidemic areas was 0.5% to 6.5%, and the average prevalence rate among populations was 1.08%. The number of echinococcosis patients was about 380,000. Echinococcosis cases were reported in 27 provinces from 2004 to 2008, with 98.2% of the reported cases distributed in seven provinces and autonomous regions including Qinghai, Tibet, Sichuan, Inner Mongolia, Gansu, Ningxia, and Xinjiang.
A survey on echinococcus was conducted among cattle and sheep slaughtered at fixed abattoirs in Xinghai County, Hainan Prefecture, Qinghai Province, from 2013 to 2014. The results showed that the average echinococcus infection rate was 7.14% in cattle and 20% in sheep (2). During the period between 2011 and 2014, the average annual infection rates of echinococcus were 26.67%, 18.94%, 11.25%, and 22%, respectively, in yaks and 46.94%, 28.94%, 12.5%, and 25.33%, respectively, in Tibetan sheep in the Guinan County of the Hainan Prefecture (3).
In 2014, a survey on the prevalence of echinococcosis was performed among 933 residents in pastoral areas in Naqu County, Tibet, showing that the prevalence rate was 8.5% among females, which was significantly higher than the rate (5.3%) among males. Hepatic echinococcosis was particularly common in people aged 40 years and over. The prevalence rate of E. granulosus was 6.2% and that of E. multilocularis was 0.6% (4).
From 2007 to 2012, the average prevalence of echinococcosis in Sichuan Province was 55.91 cases per 100,000. The incidence of the disease was highest in the 25-to-65-year-old group, and both the younger and older age groups were less affected. The ratio of male to female was 1:22. Herdsmen accounted for 84.64% among all cases, which were mainly distributed in the Ganzi Prefecture and Aba Prefecture (5). In 2012, a survey in three counties (Ruoergai County of Aba Prefecture, and Litang County and Seda County of Ganzi Prefecture) of Sichuan Province showed the average infection rate of echinococcosis was 21.27% (67/315) in cattle, 29.52% (62/210) in sheep/goats, and 14.57% (44/302) in dogs (6).
At present, the human echinococcosis cases in China have the following characteristics (7-10): (I) women are more likely to be infected, even among adolescents. The reason may be that the local women are still doing the majority of housework. The feces of infected dogs and cows disperse widely around the tents. The women collect them and make them by hand into dry dung cakes as fuel sources. They often cook while adding the dung cases to the fire. In addition, they may clean the tableware with these dung cakes. Finally, women are also responsible for all other activities such as milking, feeding dogs, and trimming sheep’s wool. Therefore, women have more direct contact with dogs, cattle, and sheep and they are at a higher risk of infection. (II) Herdsmen have higher infection rates than farmers, which may be related to their nomadic lifestyle. The herdsmen typically have a nomadic life, do long-term migration, and have a low education level, and their children have low access to school. Furthermore, they often live in poor environments and have limited access to water resources. They lack hygiene awareness and good dietary habits; in particular, they eat zanba, a Tibetan food made by hand from roasted barley flour. All the above factors increase the risk of ingesting the parasite eggs. (III) Most patients are aged between 10 and 60 years old, with the infection peak occurring at about 40 years. People in this age group engage in animal husbandry and housework more frequently and have more contact with animal hosts; in contrast, children under 10 years and old people over 60 years have fewer work activities and thus a lower risk of infection.
Clinical features
Pulmonary echinococcosis without complications can be asymptomatic. When the diameters of pulmonary hydatid cysts increase, some atypical symptoms of the lungs such as cough, chest tightness, chest pain, shortness of breath, and chest discomfort can occur. Some patients visit a local hospital due to these atypical symptoms, where cystic masses may be detected in the lungs. However, most patients seek treatment for the complications, which mainly include intracystic infection and cyst rupture. Intracystic infection can cause all infection-related symptoms such as chills, fever, and increased white blood cell count. Infection may also lead to rapid cyst enlargement in a short period of time, which can also cause symptoms such as chest pain, chest tightness, and dyspnea. Intracystic infection is often accompanied by cyst-bronchial communication, which can manifest symptoms similar to those of pulmonary abscess (e.g., coughing up odorous liquids). Cyst rupture can be divided into two types. (I) The first is rupture into bronchi where the patient may cough up clear fluid; however, asphyxia is possible if the cyst is large. In some cases, patients can even cough up white, powder-like endocyst tissue. If the patient coughs up the endocyst tissue frequently, only a round cavity may be found in the surgically resected lung specimen and the endocyst may not be visible. (II) The second type occurs when high tension makes the lung tissue under visceral pleura become extremely thin, and the cysts may break into the thoracic cavity. Because cystic fluid contains a large number of heterologous proteins secreted by hydatid, shock and even death may rapidly occur.
Diagnosis
Laboratory diagnosis
The Casoni test remains the most common means of diagnosis for echinococcosis. In the Casoni test, 0.1 to 0.3 mL of sheep hydatid cyst fluid was obtained and then intradermally injected after filtration and disinfection. A positive reaction (skin redness and swelling larger than 1/2 cm in diameter) is typically visible 5 to 10 minutes after injection. The Casoni test is simple and easy to perform, with a positive predictive value of up to 80% to 95%. However, a negative reaction does not rule out the possibility of echinococcosis, and occasionally cross-reactivity with other parasitic diseases may occur, which can lead to false positives.
Imaging
The radiographic features of pulmonary echinococcosis vary depending on the statuses of the pulmonary hydatid cysts.
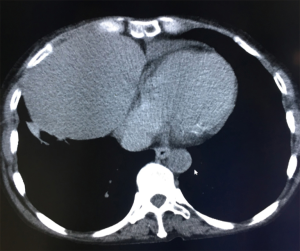
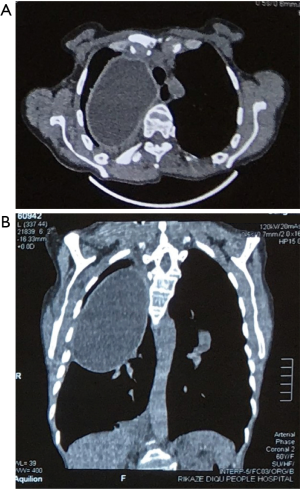
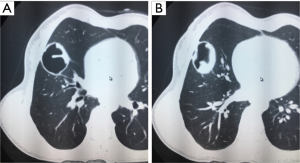
- Isodense space-occupying lesions, single or multiple, in the lungs, have been well documented for typical pulmonary hydatid cysts. The lesions are slightly more often in the right lung and are more frequently seen in the middle and lower lobes than in the upper lobe. On computed tomography (CT) scans, the density of cystic fluid is close to that of water, and the cysts have moderate wall thickness, which is larger than that of a simple pulmonary cyst. If there is an accompanying infection, the exudative changes can be seen on the outer side of the cyst wall, along with increased density of cystic fluid (Figure 3A,B).
- When the pericyst communicates with the bronchioles and the endocyst does not rupture, a small amount of air will be introduced between the pericyst and endocyst, producing the air crescent sign (Figure 4A). CT can reveal a small amount of air shadow at the inner side of the pericyst (Figure 4B).
- When the endocyst breaks and air enters parts of it, chest radiography reveals the presence of a liquid level, and the gas between the pericyst and endocyst still exists, producing parallel arches of air that are referred to as Cumbo sign or double arch sign. The double arches refer to the gas lining between the endocyst and pericyst (Figure 5).
- When the cystic fluid in the endocyst flows through its tear to the pericyst, the endocyst collapses, producing a special radiological feature known as water-lily sign or Camelotte sign (Figure 6).
- When the cystic fluid in the pericyst is completely coughed out through the airway, a gas-filled cavity can be seen in the lung, inside which the collapsed endocyst can be found (Figure 7A,B).
- When the cystic lesion is located in the right lower lung, its border with the right diaphragm is radiologically unclear, and the liver shadow is elevated. Its possible combination with hepatic echinococcosis or invasion of the diaphragm following the rupture of pulmonary/hepatic hydatid cysts should be considered. Either invasion of the right lung by liver echinococcosis or invasion of the liver by lung echinococcosis is possible.
With the help of a Casoni test and imaging methods, a diagnosis of pulmonary echinococcosis is not difficult to make if the patient comes from an endemic area of echinococcosis or a pastoral area, and/or has a typical history of canine contact, Nevertheless, radiologists and surgeons (especially those in non-epidemic areas) must be highly aware of any potential parasitic disease.
Differential diagnosis and misdiagnosis
Pulmonary echinococcosis should first of all be differentiated from a pulmonary cyst. In the absence of infection, chest X-ray has difficulty distinguishing between pulmonary echinococcosis and a pulmonary cyst. On CT, the wall of a simple pulmonary cyst is relatively thin, while the pulmonary hydatid cyst is thicker, because its pericyst is actually the compressed lung tissue. However, this difference is often neglected by doctors in non-endemic areas. Both misdiagnosed cases in the literature were from Hebei Province and Shandong Province, respectively, which are non-endemic areas for echinococcosis (11,12).
When a pulmonary hydatid cyst is accompanied by infection, symptoms such as cough and hemoptysis will occur. As the infection occurs inside the cyst, the density of cyst contents will increase to that of the soft tissue. Since lung cancer is currently the most prevalent malignant tumor, the pulmonary hydatid cyst can be easily misdiagnosed as lung cancer. In the two patients misdiagnosed as lung cancer, both had hemoptysis; one patient underwent surgery, and the other received percutaneous lung puncture before a correct diagnosis was confirmed. However, percutaneous lung puncture increased the risk of pleural hydatid cysts. Therefore, clinicians should carefully inquire about the medical history and read the CT scans before performing an invasive examination (13).
In addition, a pulmonary hydatid cyst complicated by infection needs to be differentiated from a lung abscess. Its imaging features are atypical and laboratory tests will be helpful. Active anti-infective treatment should be initiated. After timely anti-infective treatment, the lung abscess will be slowly absorbed but the pulmonary hydatid cyst will not. While surgery is indicated for a lung abscess unresponsive to antibiotics, the possibility of pulmonary echinococcosis should also be considered before the operation. The cyst should not be broken during surgery without adequate preparation (14).
Pulmonary hydatid cysts should be differentiated from encapsulated pleural effusion when they are located near the interlobar fissure. Puncture after a misdiagnosis of pleural effusion leading to pleural implantation has been reported (15).
Giant pulmonary hydatid cysts need to be differentiated from massive pleural effusion. In one report, the typical water-lily sign appeared in radiology only after the effusion was drained (16).
When a cyst ruptures, a cavity will be formed, and it is difficult to distinguished this cavity from a tuberculous cavity. Pastoral areas and semi-agricultural and semi-pastoral areas are the endemic areas of both echinococcosis and tuberculosis. Using medical imaging alone, it is sometimes difficult to differentiate a ruptured pulmonary hydatid cyst from secondary pulmonary tuberculosis. In such cases, laboratory tests such as PPD skin test, T-SPOT.TB test, and sputum stain for mycobacteria can be helpful (17).
Some other diseases may also be misdiagnosed as pulmonary echinococcosis. In one case, for example, teratoma was misdiagnosed as pulmonary echinococcosis (18). The patient lived in a pastoral area and would cough up foul-smelling sputum. Chest X-ray and CT revealed a cystic mass, with a liquefactive necrosis visible at its center. The lesion was diagnosed as a mature teratoma after surgical resection. However, a careful review of the CT scans showed that the cyst wall was obviously calcified and the density of its contents was uneven. Few reports have described cystic wall calcification in pulmonary echinococcosis cases. Thus, this diagnosis was ruled out. Another patient with a cystic mass in the right thoracic cavity was admitted to the Department of Thoracic Surgery of the Shigatse People’s Hospital, Tibet Autonomous Region in August 2017. The preoperative diagnosis was pulmonary echinococcosis and the post-operative diagnosis was type B2 thymoma, which is extremely rare. Chest CT examination revealed that although the lesion was also a cystic mass, the density of its capsule was lower than that of the pericyst of a pulmonary hydatid cyst; in fact, no obvious “pericyst” was visible on CT (Figure 8). These findings were not typical for a pulmonary hydatid cyst. Therefore, careful CT reading before a surgery is important to reduce misdiagnosis and mistreatment.
Treatment
Chemotherapy
Albendazole is a broad-spectrum anti-helminthic drug recommended by the World Health Organization (WHO) as the first choice for the treatment of echinococcosis. However, follow-up studies after oral administration of albendazole showed only 30% of patients were cured and 30–50% improved, showing unstable efficacy and tremendous inter-individual variability. The main reason may be related to the unsatisfactory absorption of the drug in the gastrointestinal tract. In recent years, the dosage forms of albendazole have been modified to improve the bioavailability of this drug. Liposomes, when used as carriers, effectively increase the drug concentrations in blood, liver, and cysts, thus improving the efficacy of albendazole in treating echinococcosis by inhibiting and killing scolices and suppressing parasite proliferation (19,20). In addition, as a routine perioperative drug, albendazole can inhibit pleural dissemination of the parasite during surgery.
Radiotherapy
Generally, radiotherapy for echinococcosis is still in the experimental stage. Sporadic reports included the use of radiosurgery with a gamma knife for treating encephalic echinococcosis in two cases (21,22) and stereotactic body radiotherapy (SBRT) for osteochondrosis in one case (23). One patient undergoing brain radiotherapy died of acute radiation injury. The symptoms were alleviated in one patient receiving bone irradiation; no germinal layer or living protoscolex was observed during puncture and drainage; after one week of culture, no growth of the scolex was observed. These studies have paved the way for future treatments.
Surgical treatment
Despite the continuous advances in medical therapies and other treatments, their effectiveness remains unsatisfactory. Thus, surgery is still the preferred treatment for pulmonary echinococcosis. The surgical principle is to remove the endocyst and its contents without contaminating other lung tissues or the pleural cavity. Although it has been proposed that another surgical principle is to maximize the preservation of lung tissue (24), we believe that removing lesions and maximizing the preservation of lung tissue are the common principles of all lung surgeries; if the cardiopulmonary conditions allow, radical resection is the top priority, and the actual function of the preserved lung tissue can also be considered.
During anesthesia, a double-lumen endotracheal tube should be used as much as possible, and inserted into the contralateral bronchus. If conditions allow, bronchoscopy can be performed after the intubation to ensure the lungs are satisfactorily separated. Lung separation can prevent suffocation and parasite migration caused by the irrigation of cystic fluid into the contralateral lung following cyst rupture.
There are three surgical methods:
- Complete excision of pulmonary hydatid cysts, including wedge resection of the lung, segmentectomy, and lobectomy. The availability of a linear cutter/stapler has made these surgeries much easier. Small lesions around the lungs can be removed by wedge resection, whereas lesions in the typical lung segments can be resected by segmentectomy. Clamping and holding the lung tissue at the lesion site using rigid instruments should be avoided as they may cause cyst rupture. This surgical method is not feasible for cysts more than 5 cm in diameter. However, chest CT-based screening for pulmonary echinococcosis is still unrealistic in epidemic areas. Therefore, most of the clinically detected pulmonary hydatid cysts are already in their later stages. Complete excision may be useful for cysts about 5 cm in diameter in middle lobe but is not feasible for other patients.
- Complete endocystectomy. The lung tissue is carefully divided from the thinnest part of the cyst surface. The gap between endocyst and pericyst is found and then bluntly separated. An anesthesiologist can also be invited to expand the lungs; tension is applied from the internal side to assist the complete removal of the endocyst. Then, the bronchi and the residual cavity are closed and drained. The linear cutter/stapler can also be applied to close the pericyst surface and remove the pericyst, during which the drainage tube of bronchiole must be closed. If necessary, several stitches can be made at pulmonary hilum to lift the tissues. It has been proposed that the best indication for complete endocystectomy is that the cyst is about 10 cm in diameter (24), However, for cyst in a single lobe, a 10-cm diameter is too large, which may increase the risk of accidental and unprepared rupture during resection and is thus more likely to cause the flow of the cyst fluid into the thoracic cavity. Therefore, we recommend the application of complete endocystectomy for cysts about 5–7 cm in diameter.
- Endocystectomy after puncture of a pulmonary hydatid cyst. When a cyst is about 10 cm or more in diameter, the risk of accidental cyst rupture can be high after complete endocystectomy. Thus, decompression through puncture is recommended before the removal of the cyst. The available techniques include the use of a puncture trocar with negative pressure suction, the application of a puncture needle, or the direct incision and suction of the endocyst, during which any potential contamination of the normal lung tissue and pleural cavity by the cystic fluid should be avoided. There are two key considerations. First, the lungs and pleura around the suction site should be protected with hypertonic saline gauze, which can effectively kill the hydatid cysts in the case that a small amount of hydatid cyst-containing cystic fluid overflows. Second, the doctor who is responsible for suction must be highly focused. Once the cystic wall is cut open, a suction device should be quickly inserted into the cyst for suction. Conditions permitting, two suction devices should be prepared. After the cyst is decompressed, 20% to 25% hypertonic saline is injected to fill the entire cyst cavity, which is soaked for 5 to 10 minutes to completely kill the scolices inside the cyst. The incision is clamped with oval forceps and the endocyst is completely removed. The outer cyst wall is then wiped with a hypertonic saline-soaked gauze to prevent cystic fluid contamination (25).
After the endocyst is removed, managing the residual cavity remains a problem. There is a consensus in the literature recommending that, for endocystectomy, the residual cavity should be ultimately sutured. However, due to the compression of the cyst, there are few residual normal lung tissues in the same lobe. For a cyst 10 cm in diameter, for example, the distance from its internal side to the large hilar vessel is typically only 3–4 cm. The pulmonary tissue on the visceral pleura side is large and thin. Even if the suture is satisfactory and the residual cavity is completely eliminated, the remaining lung tissue cannot expand satisfactorily, not to mention the postoperative complications (e.g., infection) caused by the unsatisfactory closure of the residual cavity. The above studies also did not follow up pulmonary complications or elaborate upon the expansion of residual lung tissue. Therefore, the residual cavity should be managed according to the volume of residual lung tissue and the effect of lung recruitment. When a cyst occupies more than 2/3 of the lateral side of one lung lobe, most of the remaining pulmonary tissues near the hilum are blood vessels, bronchi and alveoli occupying a small volume. Therefore, even if the closure is satisfactory and there is no residual cavity, the residual lung cannot be satisfactorily expanded. Rather, residual cavity can easily form in the thoracic cavity and lead to an increased incidence of postoperative pleural effusion and infection. In addition, due to the compression of the endocyst, most of the pulmonary tissues around the pericyst are obviously congested and thus are no longer healthy and normal pulmonary tissues. After closure, blood and gas leakage occurs easily, which increases the incidence of postoperative complications. For such patients, lobectomy can be performed after endocystectomy.
Video-assisted thoracoscopic surgery (VATS) has been a popular issue. In theory, any surgical outcomes achievable by most open surgeries are also achievable by VATS. However, VATS also has its limitations, such as the need for adequate operating space. Therefore, caution should be taken before performing VATS for large cysts. For surgical treatment, safety is always more important than efficacy. For cysts with a diameter of 5 cm or less, thoracoscopic endocystectomy or pneumonectomy can be attempted. As with open surgeries, the surrounding lungs and thoracic cavity should be protected with hypertonic saline-soaked gauzes before all operations are performed. In pace with local socioeconomic development, VATS has been gradually introduced into the endemic areas of pulmonary echinococcosis in Northwest China. Interestingly, doctors in the developed eastern regions who are more experienced in VATS have less experience in treating echinococcosis due to prevalence variation. Therefore, there is no solid evidence for the application VATS in pulmonary echinococcosis. Hospitals in Northwest China need to accumulate more experiences in VATS, so as to ensure the therapeutic effectiveness under the premise of “minimally invasive”.
Summary
Echinococcosis is the top killer of residents in pastoral areas and requires more attention. Prevention is the top priority, as the transmission route of this parasite has been well documented, with canines participating directly in the transmission to humans. Awareness-raising activities should also be enhanced. After livestock is slaughtered, its viscera should not be thrown away carelessly. Rather, they should be dealt with in a uniform way to reduce the chance of canine infection. Also, canines should be routinely administered with anthelmintics, and the definitive hosts should be well controlled, so as to reduce the chance of human infection.
Screening is another important strategy. Screening for hepatic echinococcosis has been initiated in Tibet and Xinjiang. Unlike the diagnosis of pulmonary echinococcosis, which requires chest CT, the screening for liver echinococcosis requires only a portable ultrasound machine and an ultrasound doctor. Fortunately, the Chinese government has equipped each county hospital with CT machines. In the foreseeable future, the screening for pulmonary echinococcosis will begin in many areas including Tibet, which will allow us to cure early pulmonary echinococcosis in a more minimally-invasive way.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Schad GA, Warren KS. Hookworm Disease: Current Status and New Directions. London: Taylor & Francis, 1990.
- Yuan ZY. Survey on cattle and sheep echinococcosis in Xinghai County of Qinghai Province. Shandong Journal of Animal Science and Veterinary Medicine 2015;36:52.
- Chang MH, Jin XY, Chen CY, et al. Survey on infection of echinococcosis in yaks and Tibetan sheep in Guinan County. Chinese Qinghai Journal of Animal and Veterinary Sciences 2015;45:25-6.
- Shu K, Qiong D, Wang L, et al. Incidence of liver echinococcosis in Naqu County of Tibet in 2014. Chinese Journal of Rural Medicine and Pharmacy 2015;22:63-4.
- He W, Wang Q, Huang Y, et al. Analysis of the incidence of echinococcosis in areas of Sichuan Province from 2007 to 2012. Journal of Pathogen Biology 2014;9:68-70, 91.
- Guo L, Yang AG, Zhang ZZ, et al. An epidemiological survey on livestock hydatid diseases in Sichuan Province. Chinese Journal of Veterinary Medicine 2012;48:25-7.
- Zhao YM, Jing T, Ma SM, et al. A study of the prevalence of human echinococcosis in Maqu and Luqu counties of Gannan Tibetan Autonomous Prefecture, China. Journal of Pathogen Biology 2010;5:42-3.
- Ma SM, Wang H, Zhao HL, et al. A survey on hydatidosis among females in southern Qinghai plateau. Acta Parasitologica et Medica Entomologica Sinica 2006;13:12-5.
- Feng YL, Wu XL, Li L, et al. Investigation on echinococcosis epidemiological features of female population in the rural areas of Ningxia. Modern Preventive Medicine 2011;38:3214-5, 3218.
- Cao DP, Wang H, Ma SM, et al. Epidemiological survey on human echinococcosis in Gande County of Qinghai Province. Journal of Qinghai Medical College 2000;21:7-9.
- Zhou SH, Liu LJ, Bai YS. Misdiagnosis of pulmonary echinococcosis: report of one case. Chin J Misdiagn 2007;7:6594.
- Wang ZX, Wu RQ, Wang CY. Pulmonary echinococcosis: report of one case and literature review. Journal of Clinical Pulmonary Medicine 2007;12:307.
- Zhuang L, Liu T, Wang WJ. Pulmonary echinococcosis misdiagnosed as lung cancer. Clinical Misdiagnosis & Mistherapy 2009;21:95-6.
- Zhang GH. Pulmonary echinococcosis misdiagnosed as lung cancer: Report of one case. Chinese Journal of Modern Drug Application 2012;6:99.
- Jiang CP. Clinical misdiagnosis of echinococcosis. Chinese Journal of Misdiagnostics 2002;2:142-4.
- Ciren GJ, Nima JL. Pulmonary echinococcosis misdiagnosed as pleural effusion: report of one case. Journal of Clinical Radiology 2008;27:629-30.
- Li XJ. Paerhati. X-ray misdiagnosis of cyst rupture in patients with pulmonary echinococcosis. Chinese Imaging Journal of Integrated Traditional and Western Medicine 2007;5:149-51.
- Liu YG, Li JF, Li Y, et al. Mediastinal teratoma accompanied by pleural and bronchial leakages misdiagnosed as pulmonary echinococcosis: report of one case. Chin J Thorac and Cardiovasc Surg 201026:360.
- Muhebaiti M, Liu WY, Ma WL, et al. Image findings and follow-up of lung echinococcosis after chemotherapy with liposome-entrapped albendazole. Chin J Radiol 2007;41:40-1.
- Li DB, Zhang Z. Treatment of lung echinococcosis. Journal of Xinjiang Medical University 2009;32:1621-3.
- Schmid M, Pendl G, Samonigg H, et al. Gamma knife radiosurgery and albendazole for cerebral alveolar hydatid disease. Clin Infect Dis 1998;26:1379-82. [Crossref] [PubMed]
- Zhang JJ, Zhou WL, Li JW, et al. Investigation on the acute radiation injury patient after X-ray therapy for cerebral echinococcosis. Chinese Journal of Radiological Health 2004;13:213-4.
- Xie ZJ, Bao YX, Mao R, et al. Clinical application of radiotherapy for osteochondrosis. Natl Med J China 2012;92:1932-3.
- Zhang Z, Wu MB, Zhang CM, et al. Experiences of surgical treatment in pulmonary echinococcus with entire resection of the hydatid vesicle in 292 cases. Journal of Xinjiang Medical University 2007;30:1273-4.
- Li XF, Ma JS. Surgical treatment of pulmonary echinococcus in 86 patients. Clinical Medicine of China 2010;26:851-2.