
Multiportal subxiphoid thoracoscopic major pulmonary resections
Introduction
Minimally invasive surgery has progressively been accepted, during the past decade, as the recommended treatment for early stage non-small cell lung cancer (NSCLC) (1), on the basis of a large body of literature highlighting its benefits regarding short-term outcomes, and equivalence in long-term survival compared to open surgery (2). A large spectrum of approaches, including multiportal video-assisted thoracoscopic surgery (VATS), uniportal VATS and robotic surgery, are widespreading around the world with the same major aim: to reach a safe, carcinologic resection with low morbidity. A recent randomised study (3) has confirmed VATS superiority over open surgery, for postoperative pain control and quality of life after lobectomy. However, there is still no evidence of any differences amongst the various approaches, whatever the number of ports used. Yet, reducing post-operative pain remains a daily concern for thoracic surgeons with a view to improve patient’s recovery. Therefore, there has been a growing interest in the last few years in the subxiphoid approach, aiming at minimising intercostal nerve trauma and, in consequence, reducing morbidity. Subxiphoid VATS (SVATS) has first been described for thymectomy and metastasectomy (4,5), before being extended to major pulmonary resection (MPR) in the early 2010s (6,7). More recently, a few series have also shown safe and satisfactory short-term results of both uniportal SVATS and multiportal microlobectomy (8,9). That is the spirit in which we decided to develop an original multiportal SVATS approach. This study describes and evaluates our initial experience, compared to an historic cohort of conventional VATS (CVATS).
Methods
Patient selection and study design
Seventy-five consecutive patients undergoing multiportal SVATS MPR between June 2016 and October 2017 were compared to a retrospective group of 75 consecutive patients treated by CVATS between January 2015 and May 2016. Two senior surgeons are performing MPR in our institution, one dedicated to VATS and the other to open surgery. Patients were referred indifferently to either surgeon, without considering the size or extension of tumours. Exclusion criteria for VATS were previous ipsilateral surgery and clinical stage III (major central or major chest wall extension, and N2 disease). Cardiomegaly, body mass index >30, N1 disease, centrally located tumour (visible at standard bronchoscopy) and adhesions were not considered as contraindications. The preoperative workup included computed tomographic (CT) scanning, integrated positron emission tomographic/CT scanning, and cardiopulmonary function tests. SVATS segmentectomy was indicated for ground glass opacity, cT1cN0 NSCLC with compromised pulmonary function, metastasis or small undetermined deep nodule. SVATS pneumonectomy was restricted to tumours frankly invading the fissure or the second carina, when a lung-sparing procedure was not achievable. All procedures were performed by a single general thoracic surgeon at a single tertiary French center. The study was approved by the local Ethics committee (No. 2018-033) and written informed consent was obtained from the patient after explaining the surgical procedure, for publication manuscript and accompanying images.
Surgical techniques (Figures 1,2)
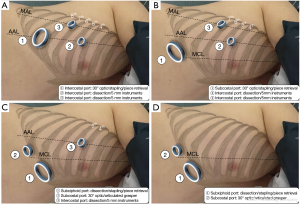
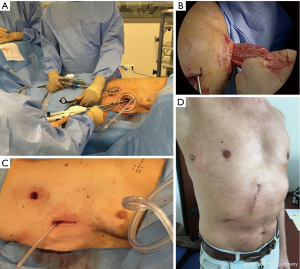
We used an anterior approach with systematic mediastinal lymph node dissection for both CVATS and SVATS. The vessels, the fissure and the bronchus were divided sequentially, with appropriate endostaplers (Covidien endo-GIA stapler with tri-staple cartridges). Thermofusion (Ligasure, Covidien) was used for dissection and small vessels. Fissure management was tailored on anatomic features, according to Walker’s classification (10): fissure-last in thick fissures grades 3–4, fissure-first occasionally in complete fissures grades 1–2 and tunnel technique, as described by Decaluwe et al. (11), in cases of thick fissures with relevant arterial anatomic variations or indication of segmentectomy.
CVATS (Figure 1A)
A 4-cm anterior utility incision was made in the 7th intercostal space (ICS) on the anterior axillary line (AAL) and protected by a wound retractor (S Alexis®, Applied Medical, USA). This access was dedicated to the 30° angled camera, stapling and specimen retrieval. Two other 10-mm ports were positioned in the 6th ICS on the middle axillary line (MAL) and the 4 h ICS on the AAL and used for dissection and occasionally for stapling, after adjunction of two small wound retractors (XXS Alexis®).
SVATS
The technical evolution was voluntarily progressive and took place in three steps:
Triportal subcostal approach (Figure 1B) for 23 patients: the 4-cm utility access was transferred under the costal arch on the medioclavicular line (MCL) through diaphragmatic muscular lateral insertions and dedicated to the 30° angled camera, stapling and specimen retrieval. Two other 10-mm ports were maintained in the 6th ICS on the MAL and the 4 h ICS on the AAL and restricted to dissection with 5-mm instruments only.
Triportal subxiphoid approach (Figure 1C) for 40 patients: the 4-cm utility access was transferred to a subxiphoid position through a paramedian vertical incision of rectus abdominis anterior aponeurosis, reaching the pleura with the finger through Larrey’s space and was dedicated to dissection, stapling and specimen retrieval. A second subcostal 15-mm port was used for the camera and an articulated grasper and one last 10-mm port in the 4th ICS on the AAL for dissection with 5-mm instruments. This approach became the standardised approach for left resection and for right complex resection.
Biportal subxiphoid approach (Figure 1D) for 12 patients: both subcostal (for the camera and an articulated grasper) and subxiphoid access (for total dissection, stapling and piece retrieval) were used with no additional intercostal port. This approach was standardised for right regular resection and was considered inadequate in left resection for safety and carcinological reasons, due to partial cardiac obstruction and a difficult access to the subcarinal area.
Postoperative management
A 24-F pleural drainage tube was placed in intercostal position for CVATS and in subxiphoid position for SVATS, and was connected to a digital suction device, with the following tube removal criteria: airflow ≤20 mL/min for at least 4h without liquid threshold except if hemorrhagic or chylous. We used for both groups an Enhanced Recovery After Surgery (ERAS) program at our institution with early nutrition and mobilisation, and a specific pain management protocol with an opioid-free intention. For our historic CVATS group, we used a complex protocol including systematic paravertebral continuous analgesia (ropivacaine) for at least 24 h, combined with oral multimodal analgesia (paracetamol, gabapentin, ketoprofen and tramadol). For the SVATS group, we used a simplified protocol associating local intercostal and subxiphoid block (ropivacaine) with oral multimodal analgesia (paracetamol, ketoprofen, tramadol). We used the Numeric Rating Scale (NRS) to evaluate pain (0–3 = mild pain; 4–6 = moderate pain; 7–10 = severe pain) and the priority goal was to keep the patient in the comfortable zone (0–3 = mild pain). If not achieved, up to four doses per day of morphine (5 mg) were given orally. Patients were discharged on the same day or the day after tube removal, if the postoperative pain was well controlled (NRS ≤3) and ambulation autonomy was recovered. All patients were reassessed by their surgeon at day 7 in the outpatient clinic and by their pneumologist at day 30.
Data collection
Data were prospectively collected and retrospectively analysed. Patients demographics, comorbidity, pulmonary function, histology, tumour size, stage, nodal status, operative characteristics and clinical outcome during hospitalisation and up to 30 days after discharge, including morbidity, mortality, length of drainage, length of stay, pain control, morphine dosage in the first 24 postoperative hours and at day 7 and readmission were evaluated. Postoperative complications were categorised according the Ottawa Thoracic Morbidity and Mortality (TMM) classification.
Statistical analysis
Data were exported from Excel to XLStat software (Microsoft Corp, Redmond, Wash). Descriptive statistics were used to estimate the frequencies of the categoric variables, medians, interquartile range (IQR), means and standard deviation of the continuous variables. Continuous variables were compared using two-tailed Mann-Whitney or Student’s t-test, as appropriate. Categorical variables were analysed by means of Fisher’s exact or chi square test.
Results
The SVATS and CVATS groups were comparable with respect to age, sex, body mass index, comorbidities and pulmonary function (Table 1). Tumour histology, size, stage and nodal status were also comparable between both groups (Table 2), with a R0 resection achieved for all patients with NSCLC.
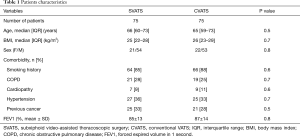
Full table
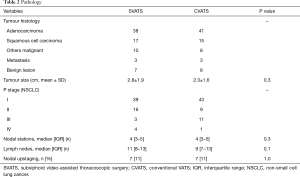
Full table
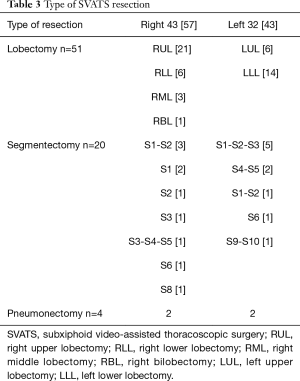
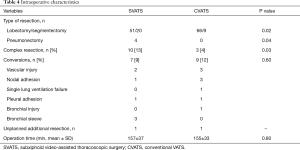
Table 3 illustrates the panel of MPRs performed by SVATS. Regarding intraoperative characteristics (Table 4), the lobectomy/segmentectomy ratio was significantly higher in the CVATS group (P=0.02), while the SVATS group included significantly more pneumonectomies (P=0.04). Complex resections were significantly more frequent in the SVATS group (13% vs. 4%, P=0.03): 4 pneumonectomies, 3 sleeve lobectomies including 2 planned conversions for the bronchial anastomotic step and 1 unplanned, 1 segmentectomy with chest wall resection, 1 hybrid Pancoast right upper lobectomy with en bloc first rib and sleeve subclavian artery resection, 1 S1 extra-pleural segmentectomy with subclavian arteriolysis for aspergilloma and 1 major adhesiolysis, whereas only 2 intrapericardial lobectomies and 1 lobectomy with chest wall resection were noted in the CVATS group.
Full table
Full table
Operation time and conversion rate were statistically similar in both groups (Table 4). Two vascular injuries in the SVATS group were managed by sponge stick compression and non-urgent anterior thoracotomy (300 mL blood loss each) and discharged on day 1, without blood transfusion. One unplanned additional resection was noted in each group (a sleeve left lower lobectomy for an erroneous stapling of main stem bronchus by SVATS and a right lower bilobectomy after a bronchus intermediate injury during a lower lobectomy by CVATS).
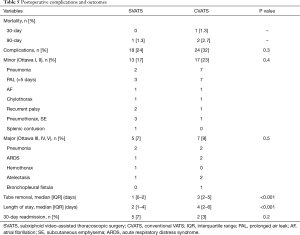
There was no 30-day mortality in the SVATS group and a 1.3% mortality in the CVATS group (Table 5). Overall complication rates were not statistically different (24% vs. 32%). The SVATS group had a significantly shorter length of drainage (median: 1 vs. 3 days, P<0.001), and postoperative length of stay, (median: 2 vs. 4 days, P<0.001). 30-day readmission rates did not differ between both groups. Pneumonectomies had an uneventful follow-up and there was no subxiphoid hernia.
Full table
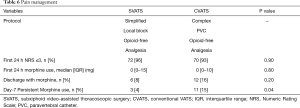
Regarding postoperative pain (Table 6), there was no difference between SVATS and CVATS in the first postoperative 24 h, in terms of pain control (NRS ≤3: 96% vs. 93%) and morphine use (median 0 mg in each). It should be noted that both groups had in common an oral multimodal opioid-free analgesia but differed significantly in terms of locoregional pain management protocol: the CVATS group received a paravertebral catheter continuous analgesia, during a median of 2 days, whereas the SVATS group received a local one-shot subxiphoid and intercostal block. The SVATS group presented a trend toward less patients discharged home with morphine (8% vs. 16%, P=0.2) and significantly less patients with persistent morphine use necessary to maintain them in the comfortable zone (NRS ≤3) at day 7 (4% vs. 15%, P=0.04).
Full table
Discussion
This study shows that multiportal SVATS MPR is safe and achieves R0 resection for NSCLC with complete lymphadenectomy. Since the first case reports (6,7), a few recent series have demonstrated the feasibility of subxiphoid MPR with satisfactory short-term results. Hernandez et al. (8) and Aresu et al. (12) from Shanghai hospital reported in 2016 the effectiveness of both uniportal SVATS lobectomy in 153 selected patients, and uniportal SVATS segmentectomy in 84 patients. Dunning et al. (9) reported in 2017 a multicenter selected series with satisfactory outcomes, through a totally portal subxiphoid assisted “microlobectomy” in 72 patients. These different approaches have in common the pursuit of minimal invasiveness, for which, in our view, location of ports could matter more than number.
We present in this study our initial experience in SVATS, that was conceived in a multiportal way and designed gradually to guarantee safety. The conversion rate was low (7 patients including 2 planned sleeve lobectomies, 3 technical difficulties and 2 vascular injuries), despite a non-selected consecutive series with a substantial proportion of complex resections, indicating a good level of reproducibility. Subxiphoid relative distance to the operative field was integrated in our routine policy to prevent major intraoperative events (13,14) so that the 2 vascular injuries observed were controlled and converted in a non-urgent manner using well-defined strategies, as already described in completely portal robotic surgery (15).
The key element of this subxiphoid approach is to avoid intercostal nerve damage by minimising the lever effect on the ribs. To this end, the ports located under the subcostal arch are dedicated to the larger elements (endostaplers, camera, specimen removal and chest tube) while the intercostal port, if necessary, is restricted to 5-mm instruments. We can highlight several benefits of this technique: high manoeuvrability of the endostapler, panoramic view from above on both anterior and posterior mediastinum with a subcostal 30° camera, limitation of conflicts between camera and other instruments due to the duplicated access, intuitive and safe dissection from the subxiphoid access, easy subxiphoid specimen removal even for tumours up to 9 cm and painless subxiphoid chest tube. Limitations include the inability to palpate the lung, and a relatively longer learning curve for left side resections. Therefore, a triportal approach was maintained on the left side for safety and carcinologic reasons, due to partial cardiac obstruction and relatively difficult access to the subcarinal area, as described in uniportal SVATS (8,12), whereas an exclusive subxiphoid biportal approach was standardised for right side resections.
Multiportal SVATS appeared appropriate for lobectomy but also for both segmentectomy and pneumonectomy. SVATS pneumonectomy allowed a tension-free subxiphoid piece retrieval, offering in our view an important benefit on postoperative pain and recovery, which is not observed with CVATS (16).
In our study, we compared the outcome of our patients undergoing SVATS MPR, against an historic cohort of CVATS. Our results show a significantly shorter length of drainage, a shortened hospitalisation and a significant reduction in persistent morphine use at day 7 for SVATS patients, without any difference in terms of morbidity, mortality and readmission rate, suggesting a trend for better recovery in the SVATS group. The shortened hospitalisation observed is partially due to the shortened length of drainage, that could have been influenced by the experience gained during the study in digital drainage device management. However, SVATS was particularly appropriate for a tailored fissure management with few prolonged air leak (4%).
Very limited data are available regarding the effect of SVATS on postoperative pain after thoracic surgery. Suda et al. suggested, in a retrospective study, that patients use less morphine after subxiphoid thymectomy than after CVATS (17). In our clinical practice, the priority daily goal is to maintain the patient comfortable (NRS ≤3) and analgesia is adjusted in consequence, with an opioid-free intention. This goal was equally achieved in both SVATS and CVATS groups in the first 24 hours, with use of a significantly simplified protocol for SVATS patients, and our study suggests a superiority of SVATS concerning persistent morphine use at day 7. The clinical relevance of these results has however to be asked, especially when comparing two minimally invasive approaches.
Nevertheless, post-thoracotomy pain syndrome remains a problem in thoracic surgery, impairing quality of life after both thoracotomy and VATS, with a prevalence of approximately 25–50%, and 10% of patients experiencing debilitating pain (18). In most countries, the standard of care is to prescribe opioids after discharge, leading to a significant public health issue of potentially dramatic long-term opioid use: up to 20% after thoracotomy, significantly reduced to 11% after VATS, as described recently (19). From that perspective, the level of compliance to opioid-free analgesia observed in our study, particularly in the SVATS group, suggests the superiority of this minimally invasive approach.
This study has several weaknesses: it is a single institutional study and the SVATS approach was slightly modified during the study. Furthermore, the control CVATS group was an historic group retrospectively assessed without applying a propensity-matched analysis. Bias potentially occurred because of changes in pain management protocol and the reinforcement of our ERAS program during the study, which could have an impact on postoperative length of stay regardless of the approach. Data are also lacking about pain management 30 days after discharge. Considering its strengths, we compared two consecutive series of non-selected patients operated on by a single surgeon.
In conclusion, this study shows that multiportal SVATS is a safe and efficient approach for MPR. Subxiphoid approaches are very recent and their clinically relevant interest has naturally to be assessed in larger studies. We still believe that the multiportal nature of our subxiphoid approach gives it its strength, allowing a gradual acquisition of the technique, without any rupture in VATS paradigm, and preserving safety and carcinologic imperatives. We could also see in the near future, as already anticipated (20-22), the establishment of subxiphoid robotic surgery, combining high precision and minimal invasiveness.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the local Ethics committee (No. 2018-033) and written informed consent was obtained from the patient after explaining the surgical procedure, for publication manuscript and accompanying images. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Howington J, Blum M, Chang A, et al. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd edition. American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:278-313.
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or antero- lateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Mineo TC, Pompeo E, Ambrogi V, et al. Video-assisted approach for transxiphoid bilateral lung metastasectomy. Ann Thorac Surg 1999;67:1808-10. [Crossref] [PubMed]
- Kido T, Hazama K, Inoue Y, et al. Resection of anterior mediastinal masses through an infrasternal approach. Ann Thorac Surg 1999;67:263-5. [Crossref] [PubMed]
- Oda M, Matsumoto I, Waseda R, et al. Total port-access lobectomy via a subcostal trans-diaphragmatic approach for lung cancer. Interact Cardiovasc Thorac Surg 2013;16:211-3. [Crossref] [PubMed]
- Liu CC, Wang BY, Shih CS, et al. Subxiphoid single-incision thoracoscopic left upper lobectomy. J Thorac Cardiovasc Surg 2014;148:3250-1. [Crossref] [PubMed]
- Hernandez-Arenas LA, Lin L, Yang Y, et al. Initial experience in uniportal subxiphoid video-assisted thoracoscopic surgery for major lung resections. Eur J Cardiothorac Surg 2016;50:1060-6. [Crossref] [PubMed]
- Dunning J, Elsaegh M, Nardini M, et al. Microlobectomy: A Novel Form of Endoscopic Lobectomy. Innovations (Phila) 2017;12:247-53. [Crossref] [PubMed]
- Craig SR, Walker WS. A proposed anatomical classification of the pulmonary fissures. J R Coll Surg Edinb 1997;42:233-4. [PubMed]
- Decaluwe H, Sokolow Y, Deryck F, et al. Thoracoscopic tunnel technique for anatomical lung resections: a 'fissure first, hilum last' approach with staplers in the fissureless patient. Interact Cardiovasc Thorac Surg 2015;21:2-7. [Crossref] [PubMed]
- Aresu G, Weaver H, Wu L, et al. The Shanghai Pulmonary Hospital uniportal subxiphoid approach for lung segmentectomies. J Vis Surg 2016;2:172. [Crossref] [PubMed]
- Flores RM, Ihekweazu U, Dycoco J, et al. Video-assisted thoracoscopic surgery (VATS) lobectomy: catastrophic intraoperative complications. J Thorac Cardiovasc Surg 2011;142:1412-7. [Crossref] [PubMed]
- Decaluwe H, Petersen RH, Hansen H, et al. Major intra- operative complications during video-assisted thoracoscopic anatomical lung resections: an intention-to-treat analysis. Eur J Cardiothorac Surg 2015;48:588-98. [Crossref] [PubMed]
- Cerfolio RJ, Bess KM, Wei B, et al. Incidence, Results, and Our Current Intraoperative Technique to Control Major Vascular Injuries During Minimally Invasive Robotic Thoracic Surgery. Ann Thorac Surg 2016;102:394-9. [Crossref] [PubMed]
- Battoo A, Jahan A, Yang Z, et al. Thoracoscopic pneumonectomy: an 11-year experience. Chest 2014;146:1300-9. [Crossref] [PubMed]
- Suda T, Hachimaru A, Tochii D, et al. Video-assisted thoracoscopic thymectomy versus subxiphoid single-port thymectomy: initial results†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i54-8. [PubMed]
- Wildgaard K, Ravn J, Nikolajsen L, et al. Consequences of persistent pain after lung cancer surgery: a nationwide questionnaire study. Acta Anaesthesiol Scand 2011;55:60-8. [Crossref] [PubMed]
- Tuminello S, Schwartz RM, Liu B, et al. Opioid Use After Open Resection or Video-Assisted Thoracoscopic Surgery for Early-Stage Lung Cancer. JAMA Oncol 2018;4:1611-3. [Crossref] [PubMed]
- Ninan M, Dylewski M. Total port-access robot-assisted pulmonary lobectomy without utility thoracotomy. Eur J Cardiothorac Surg 2010;38:231-2. [Crossref] [PubMed]
- Suda T. Robotic subxiphoid thymectomy. J Vis Surg 2016;2:118. [Crossref] [PubMed]
- Nardini M, Migliore M, Jayakumar S, et al. Subxiphoid port applied to robotic pulmonary lobectomies. J Vis Surg 2017;3:35. [Crossref] [PubMed]


