Comparing the benefits of postoperative adjuvant chemotherapy vs. observation for stage IB non-small cell lung cancer: a meta-analysis
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide, and non-small cell lung cancer (NSCLC) accounts for approximately 80% of the lung carcinoma cases (1). Treatments for NSCLC patients include surgery, chemotherapy, radiotherapy, and targeted therapy. Fortunately, a randomized controlled trial (RCT) has shown that resected NSCLC patients benefit from adjuvant chemotherapy (2). However, the use of postoperative adjuvant chemotherapy for stage IB NSCLC in the setting of standard therapy remains controversial (3).
Some previous studies have reported that stage IB NSCLC patients should be treated with adjuvant chemotherapy (4,5). In contrast, other studies reported that there was no benefit of postoperative adjuvant chemotherapy (6). Using different guidelines, the European Society for Medical Oncology Clinical Practice Guidelines (7) suggested that adjuvant chemotherapy could be considered for patients with resected stage IB disease. However, according to the National Comprehensive Cancer Network (NCCN) guidelines (8), there is a lack of a standard to precisely assign chemotherapy to stage IB NSCLC patients. Typical high-risk factors in stage IB NSCLC patients include poorly differentiated tumors, vascular invasion, wedge resection, tumor sizes >4 cm, visceral pleural infiltration, and incomplete lymph node sampling. However, independent indication may not be used as an effective standard, but should be used only as a reference factor. Based on different guidelines, the use of postoperative adjuvant chemotherapy for stage IB NSCLC patients is still controversial. A meta-analysis was therefore performed to quantify the prognostic differences between stage IB NSCLC patients with postoperative adjuvant chemotherapy vs. observation, which should provide more reliable and updated evidence to treat resected stage IB NSCLC patients.
Methods
Literature search
A systematic literature search was conducted for the following terms: “lung cancer” (MeSH Terms) AND “adjuvant chemotherapy” (MeSH Terms) AND “surgery” (MeSH Terms), and was used to achieve the maximum sensitivity in the electronic PubMed, Embase, and Cochrane Library databases from the earliest publications to June 2018.
Selection criteria
The criteria for inclusion were as follows: (I) resected NSCLC patients who received adjuvant chemotherapy or observation were eligible for inclusion; (II) an accurate p-stage IB (T2N0M0) NSCLC based on the 6th (same as 5th edition) and 7th edition of the Union for International Cancer Control Tumor Node Metastasis classification; and (III) all articles were published in English. The following studies were excluded: (I) NSCLC patients who received preoperative or any other postoperative antitumor treatments; (II) case reports, expert opinions, abstracts, conference presentations, guidelines, reviews, and low quality studies (Grade C); (III) data which could not be extracted from the literature; and (IV) if repeated results were chosen, the study with the smallest sample was excluded.
Data extraction
Data from eligible studies were independently extracted in a standardized manner by two inspectors (R Li and G Yang). The data extraction included the following: first author, research type, time of publication, numbers of patients with 5-year overall survival (OS), 5-year disease-free survival (DFS), local recurrence, and distant metastasis. Discrepancies in data extraction were resolved by consensus with a senior inspector (Y Tian), and the study design and critical review was provided by the senior investigator (D Tian).
Quality evaluation
The Cochrane Collaboration’s tool for assessing risk of bias was used in RCTs including seven areas of the project team. Each indicator was judged by using “low risk of bias” “high risk of bias”, and “uncertain risk of bias”. For each study, if the conditions of the study were consistent with the seven areas of the project team, the study was regarded as a high quality study (Grade A); if the conditions of the study were partially consistent with the project team, the study was regarded as a medium quality study (Grade B); and the study was regarded as a low quality study (Grade C) when there was little consistency with the seven areas of project team.
Statistical analysis
All statistical analyses were conducted using Review Manager 5.3 software. The I2 test was used to appraise the heterogeneity in the meta-analysis. If the heterogeneity was low (I2 ≤50%), the fixed-effects model was used. Otherwise, the random-effects model was used (I2 >50%). Sensitivity analyses and subgroup analyses were used to evaluate the sources of heterogeneity. The evaluation of publication bias was analyzed using the funnel plot, and differences were considered significant at a value of P<0.05.
Results
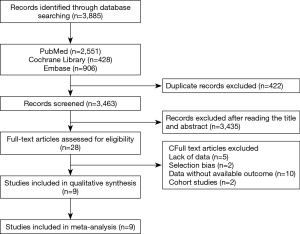
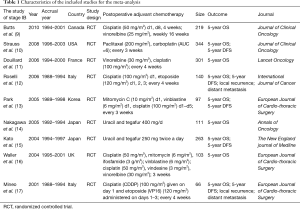
A total of 3,885 potential articles were retrieved from electronic databases. Finally, a total of nine RCTs (9-17) (Figure 1, Table 1) were obtained. The meta-analysis involved 1,645 patients who were assigned to the adjuvant chemotherapy (n=820) and observation (n=825) groups. Included in the meta-analysis were nine studies describing the 5-year OS, five studies describing the 5-year DFS, and two studies describing local recurrence and distant metastasis.
Full table
The 5-year OS
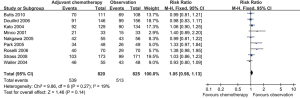
There was no significance in the 5-year OS [relative risk (RR) =1.05; 95% confidence interval (CI): 0.98–1.13; P=0.14] (Figure 2) between postoperative adjuvant chemotherapy versus observation. There was low heterogeneity, so the fixed-effects model was used.
Five-year DFS
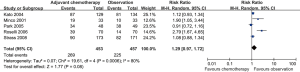
There was no significant difference in the 5-year DFS (RR =1.29; 95% CI: 0.97–1.72; I2 =80%; P=0.08) (Figure 3) between postoperative adjuvant chemotherapy versus observation, and there was substantial heterogeneity, so the random-effects model was used.
Local recurrence
There was a significant difference in the local recurrence (RR =0.43; 95% CI: 0.23–0.80; I2 =28%; P=0.007) (Figure 4) between the postoperative adjuvant chemotherapy and observation groups, and there was low heterogeneity, so the fixed-effects model was used.
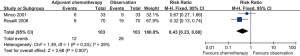
Distant metastasis
Distant metastasis (RR =0.68; 95% CI: 0.48–0.97; I2 =0%; P=0.03) (Figure 5) was significantly different in the postoperative adjuvant chemotherapy vs. observation groups, and as there was low heterogeneity, so the fixed-effects model was used.

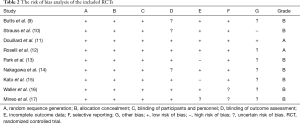
The analysis of methodological quality
The Cochrane Collaboration’s tool was used for RCTs (Table 2).
Full table
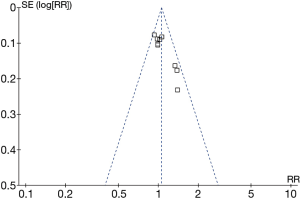
The analysis of sensitivity analysis and publication bias
The sensitivity test was conducted by successively excluding individual studies and recalculating the consequences. However, the source of heterogeneity was not confirmed by using sensitivity analysis in the analysis of the 5-year DFS. The evaluation of publication bias was analyzed by the funnel plot, which was an asymmetrical funnel diagram when the data were biased. The analysis showed that multiple points were evenly distributed on both sides of the longitudinal axis, so there was no obvious publication bias (Figure 6).
Discussion
According to NCCN guidelines, the value of adjuvant chemotherapy has been proven by multiple large randomized trials for resected stage II and IIIA NSCLC patients, but the role of adjuvant chemotherapy is controversial for stage IB patients. The objective of this meta-analysis was therefore to assess the therapeutic effect of adjuvant chemotherapy vs. observation on resected stage IB NSCLC patients. The data included the 5-year OS, 5-year DFS, local recurrence, and distant metastasis.
We found two positive retrospective studies (18,19), which were designed specifically for stage IB NSCLC patients; both recommended adjuvant chemotherapy for these patients. However, the use of non-randomized studies was of minimal value and the results of these studies were more relevant to high risk stage IB NSCLC patients. Although impressive, the small sample size and the large effect size prompted questions about the reproducibility of these findings. Because they might cause selection bias and reduce the scientific strength of the meta-analysis, we excluded these two retrospective studies. Nine RCTs, including the 5-year OS and seven RCTs, including the 5-year DFS were finally included. Three studies in the 5-year OS and two studies in the 5-year DFS supported efficacy for adjuvant chemotherapy in stage IB NSCLC patients, but these originated from small RCTs. Conversely, other RCTs did not indicate a clear benefit of adjuvant chemotherapy. The meta-analysis found updated results showing no significance in the 5-year OS, including 1,645 patients and a 5-year DFS, by comparing 910 patients involved in postoperative adjuvant chemotherapy and observation. Obviously, adjuvant chemotherapy is not recommended as a standard of therapy in stage IB NSCLC patients. The outcome was consistent with NCCN guidelines, which indicated a lack of an effective standard to precisely assign chemotherapy to stage IB NSCLC patients.
Remarkably, based on the results of previous meta-analyses, Burdett et al. (20) reported that platinum-based adjuvant chemotherapy in patients with stage IB tumors was similar to estimates for patients with stage II and III tumors. However, it was found that the relative effect of adjuvant chemotherapy did not differ significantly by stage in their meta-analysis. Although application of the overall hazard ratio to survival in the control group by stage suggested absolute improvements in 5-year survival of 5% of the patients with stage IB (from 55% to 60%), there were no significance between postoperative adjuvant chemotherapy and observation in stage IB NSCLC patients. These results differed from our conclusion, which might be explained by the eligibility criteria for inclusion involved in the p-stage I to IV disease in the previous meta-analysis. However, the criteria for inclusion in our meta-analysis was p-stage IB (T2N0M0) NSCLC, so it was a more targeted analysis.
Two RCTs described data of local recurrence and distant metastasis. The results showed that stage IB NSCLC patients might receive a benefit from adjuvant chemotherapy to reduce risks of local recurrence and distant metastasis after surgery. It might be interpreted that the value of postoperative adjuvant chemotherapy could reduce risks of recessive micrometastases (tumor cell deposition in the lymph tissue of 1/6 of the patients deposited at 0.2–2 mm), which might generate recurrence or metastasis after tumor resection (21). However, more attention should be directed to the fact that only 206 patients were included in local recurrence and distant metastasis in the meta-analysis, and the number of positive events was small. These results might be influenced by sample number in the meta-analysis. Although we concluded a positive result, there was not enough evidence to recommend adjuvant chemotherapy for stage IB patients, so we expect that additional RCTs will contribute more reliable results to assess whether adjuvant chemotherapy clearly reduces the risks of local recurrence and distant metastasis.
In addition, Strauss recommend considering patients with stage IB disease and larger tumors (≥4 cm) for cisplatin-based adjuvant chemotherapy on an individualized basis. In previous studies, there has been controversy about the effect of adjuvant chemotherapy for stage IB NSCLC patients with 4 cm tumor sizes (22,23). However, there is currently insufficient data concerning stage IB and tumor size, and the size of tumors is outside of the objectives of our meta-analysis, so we will evaluate the effect of adjuvant chemotherapy for stage IB tumor size in the future.
Our meta-analysis had several limitations. There was a limitation of sample size in the study. In addition, the patients’ individual data included sex, age, smoking, type of pathology, differentiation, tumor size, type of chemotherapy, tumor marker, and drug toxicity. If the data were available, we might perform a more comprehensive guideline study for stage IB NSCLC patients in the future and could find the sources of heterogeneity.
Conclusions
The 5-year OS and 5-year DFS of stage IB NSCLC patients were not improved by adjuvant chemotherapy. In addition, there was not enough evidence to show that adjuvant chemotherapy reduced the risks of local recurrence and distant metastasis after surgery, because these results might be influenced by sample size in the meta-analysis. In conclusion, adjuvant chemotherapy might not be recommended for stage IB NSCLC patients.
Acknowledgments
We would like to thank Dali Tian, at The Fourth Affiliated Hospital of China Medical, for study design and critical review.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All data in this study were obtained from databases, so ethical approval and informed consent are not required.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Su S, Scott WJ, Allen MS, et al. Patterns of survival and recurrence after surgical treatment of early stage non-small cell lung carcinoma in the ACOSOG Z0030 (ALLIANCE) trial. J Thorac Cardiovasc Surg 2014;147:747-52; discussion 752-3. [Crossref] [PubMed]
- Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 2005;352:2589-97. [Crossref] [PubMed]
- Bunn PA Jr. Early-stage non-small-cell lung cancer: current perspective in combined-modality therapy. Clin Lung Cancer 2004;6:85-98. [Crossref] [PubMed]
- Dediu M, Ion O, Ion R, et al. Impact of adjuvant chemotherapy in stage IB non-small-cell lung cancer: an analysis of 112 consecutively treated patients. J BUON 2012;17:317-22. [PubMed]
- Heon S, Johnson BE. Adjuvant chemotherapy for surgically resected non-small cell lung cancer. J Thorac Cardiovasc Surg 2012;144:S39-42. [Crossref] [PubMed]
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-21. [Crossref] [PubMed]
- Ettinger DS, Aisner DL, Wood DE, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5 2018. J Natl Compr Canc Netw 2018;16:807-21. [Crossref] [PubMed]
- Butts CA, Ding K, Seymour L, et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: updated survival analysis of JBR-10. J Clin Oncol 2010;28:29-34. [Crossref] [PubMed]
- Strauss GM, Herndon JE 2nd, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 2008;26:5043-51. [Crossref] [PubMed]
- Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006;7:719-27. [Crossref] [PubMed]
- Roselli M, Mariotti S, Ferroni P, et al. Postsurgical chemotherapy in stage IB nonsmall cell lung cancer: Long-term survival in a randomized study. Int J Cancer 2006;119:955-60. [Crossref] [PubMed]
- Park JH, Lee CT, Lee HW, et al. Postoperative adjuvant chemotherapy for stage I non-small cell lung cancer. Eur J Cardiothorac Surg 2005;27:1086-91. [Crossref] [PubMed]
- Nakagawa M, Tanaka F, Tsubota N, et al. A randomized phase III trial of adjuvant chemotherapy with UFT for completely resected pathological stage I non-small-cell lung cancer: the West Japan Study Group for Lung Cancer Surgery (WJSG)—the 4th study. Ann Oncol 2005;16:75-80. [Crossref] [PubMed]
- Kato H, Ichinose Y, Ohta M, et al. A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med 2004;350:1713-21. [Crossref] [PubMed]
- Waller D, Peake MD, Stephens RJ, et al. Chemotherapy for patients with non-small cell lung cancer: the surgical setting of the Big Lung Trial. Eur J Cardiothorac Surg 2004;26:173-82. [Crossref] [PubMed]
- Mineo TC, Ambrogi V, Corsaro V, et al. Postoperative adjuvant therapy for stage IB non-small-cell lung cancer. Eur J Cardiothorac Surg 2001;20:378-84. [Crossref] [PubMed]
- Jang HJ, Cho S, Kim K, et al. Effect of Adjuvant Chemotherapy after Complete Resection for Pathologic Stage IB Lung Adenocarcinoma in High-Risk Patients as Defined by a New Recurrence Risk Scoring Model. Cancer Res Treat 2017;49:898-905. [Crossref] [PubMed]
- Park SY, Lee JG, Kim J, et al. Efficacy of platinum-based adjuvant chemotherapy in T2aN0 stage IB non-small cell lung cancer. J Cardiothorac Surg 2013;8:151. [Crossref] [PubMed]
- Burdett S, Pignon JP, Tierney J, et al. Adjuvant chemotherapy for resected early-stage non-small cell lung cancer. Cochrane Database Syst Rev 2015.CD011430. [PubMed]
- Tezel C, Ersev AA, Kiral H, et al. The impact of immunohistochemical detection of positive lymph nodes in early stage lung cancer. Thorac Cardiovasc Surg 2006;54:124-8. [Crossref] [PubMed]
- Morgensztern D, Du L, Waqar SN, et al. Adjuvant Chemotherapy for Patients with T2N0M0 NSCLC. J Thorac Oncol 2016;11:1729-35. [Crossref] [PubMed]
- Malhotra J, Mhango G, Gomez JE, et al. Adjuvant Chemotherapy for elderly patients with stage I nonf-Small cell lung cancer ≥4 cm in size: A SEER-Medicare Analysis. Ann Oncol 2015;26:768-73. [Crossref] [PubMed]