Surgical technique modifies the postoperative atrioventricular block rate in sutureless prostheses
Introduction
The Perceval S biological sutureless aortic prosthesis (LivaNova, Saluggia, Italy) represents an alternative to conventional bioprostheses in aortic valve replacement (AVR). This is a prosthesis specifically designed to be implanted using minimally invasive approaches (1). It presents excellent haemodynamic results, especially in small roots and allows a reduction in surgical time thereby making it into an alternative to AVR in medium-high risk patients (2). This is why its use in AVR has spread. It has, however, been associated with a high incidence of postoperative complete atrioventricular block (CAVB) which requires the implantation of a permanent pacemaker (PPM). This complication has been described in the scientific literature with great variability, 8–23%, far higher than those obtained in AVR with conventional bio prostheses (3).
The main objective was to analyse the impact of the modification of the Perceval S implantation technique on the prevalence of postoperative atrioventricular block (AVB) which requires a PPM in our series. The secondary objectives were to identify the risk factors that influence the appearance of this complication.
Methods
Patients
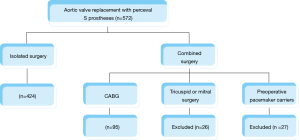
From October 2013 to July 2018, 572 AVRs were performed in our centre with Perceval S prostheses.
We excluded patients undergoing valve surgery that involved any mitral or tricuspid procedure as well as patients carrying definitive pacemakers prior to the surgery (Figure 1). The study was approved by the local ethics committee. All patients recruited signed their informed consent.
A descriptive, retrospective, non-randomised study was performed in our series. All patient data was collected in the database of the department. We collected the preoperative characteristics of the patients, including disorders in intraventricular cardiac conduction prior to the operation. We collected the characteristics of the procedure and times of the operation as well as the complications and postoperative hospital stay intervals. Main outcome measured was PPM implantation in patients with postoperative AVB occurred during hospitalization period. Implant was carried out at least 15 days far from the surgery (following cardiologist recommendations) to ensure there were no rhythm recovery, but always during surgery hospitalization period, prior to hospital discharge. An active follow-up of the implanted pacemakers was carried out during the postoperative period in the hospital environment, at one month and at one year from implantation. Electrophysiological variables of interest were collected.
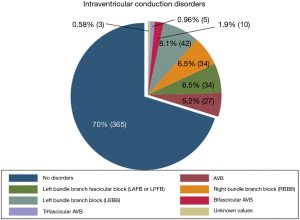
Six categories were set up based on the disorders of the intraventricular conduction prior to surgery. Patients who did not have any conduction disorder, patients who had only first degree atrioventricular block (first degree AVB). Patients with either isolated left anterior or posterior fascicular block (LAFB or LPFB). Patients with complete left bundle branch block (LBBB). Patients with isolated right bundle branch block (RBBB). Patients with bifascicular block (BFB) composed of a first degree AVB associated with a RBBB or LAFB/LPFB. Finally, patients with trifascicular block (TFB) composed of patient who had a first degree AVB together with an RBBB and a LAFB/LPFB or, first degree AVB together with a LBBB (in electrophysiological terms, the trifascicular block is equivalent to a complete atrioventricular block or alternating bundle branch block, although in this case, the definition of this study aims to group the categorical variables of interest and facilitate the understanding of the same).
Two subgroups were set up for sample analysis (n) in accordance with the surgical technique used. The subgroups were successive over time and were subject to the exclusion criteria cited.
Surgery
The patients underwent isolated AVR surgery, in which as a minimally invasive approach a partial sternotomy in J through the third intercostal space was used or a full sternotomy (according to the criterion of the operating surgeon), or combined aortic valve replacement surgery [AVR combined with coronary artery bypass graft (CABG) surgery] approached through a full sternotomy. Aortic valve replacement was done using the conventional technique with aortic cross-clamp, use of antegrade and/or retrograde blood cardioplegia and high transversal aortotomy.
The implantation of the prosthesis was done using two different techniques. Between October 2013 and November 2016 the standard technique was used (4). The specific aortic annular gauges of the prosthesis were used. The prosthesis was taken down folded guided by 3 points made in the respective nadirs with an amplitude of 2–3 mm on both sides of the valvular annulus. Without entering the outflow tract of the left ventricle (LV) the prosthesis was unfolded on the annular level and a ballooning was conducted at four atmospheric pressures for 30 seconds. In the initial phase of the programme a subtle annular decalcification was used and subsequently a more rigorous decalcification.
Our group modified the implantation technique in November 2016, operating from then until July 2018 n=302 patients with the modified technique. A rigorous decalcification of the aortic annulus was performed. The annular measurement was modified, such that we decided to choose the prosthetic size whose opaque gauge has a maximum prosthetic diameter and that could therefore be introduced into the outflow tract of the IV in a way similar to the measurement of conventional bioprostheses (thereby avoiding the oversizing of the annular and the underexpansion or deformation of the prosthesis in deployment). Our group introduced the folded prosthesis through the valvular annulus until it crossed the outflow tract of the IV and then they repositioned the same in the annulus by traction of the 3-point guide, with lower amplitude (1–2 mm). Finally, a short-duration ballooning was performed, of less than 5 seconds and reaching the four atmospheric pressures (4).
Statistical analysis
A descriptive analysis was made of all the variables included in the study. A normality test was performed for continuous variables using the Saphiro-Wilk test. The quantitative variables were expressed as the mean (standard deviation) or median (range) as appropriate. The qualitative variables were expressed as a percentage y (absolute value n). The means were compared by Student’s t-test or Mann-Whitney test, as appropriate. The differences between group variables were analysed using the Student t-test for independent data. The association of qualitative variables was estimated by means of the chi-square or Fisher statistic. A value of P<0.05 was considered statistically significant.
The risk factors for the presence of postoperative AVB were assessed by analysing univariate and multivariate logistic regression. A regression model of an explanatory nature was set up in the multivariate analysis taken from all the possible models based on the variables associated with postoperative AVB in the scientific literature. The maximum model included the variables, technique used, height (cm), body surface area (m2), reoperation, presence of endocarditis, size of the implanted prosthesis, prior atrial rhythm, intraventricular conduction disorders prior to the operation, first order interactions and interaction of height with implanted prosthesis size which was considered to be of interest for the study. The categorical variables took as a reference respectively the standard technique, non-reoperation, absence of endocarditis, sinus rhythm and absence of intraventricular conduction disorders. The best model was chosen according to the criterion of the Akaike information criterion (AIC), Bayesian information criterion (BIC), Hosmer-Lemeshow test and predictive ability of the model (AUC). We calculated the regression coefficients and their respective Odds Ratio (OR).
The missing values were treated statistically as unknown values.
The StataCorp software was used. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP for the statistical analysis.
Results
Table 1 includes the results corresponding to the pre-surgical characteristics. Major demographic variables in our series, Euroscore I and II, presence of cardiovascular risk factors, major comorbidities and characteristics of the echocardiography prior to the operation.
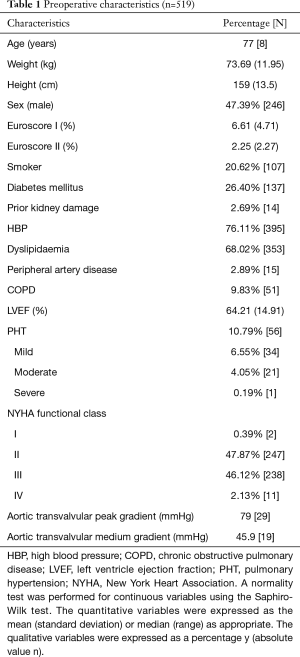
Full table
Among the intraoperative characteristics of patients, Table 2 includes the type of surgical approach, the patients previously operated with AVR or sufferers of endocarditis with surgical indication, the distribution of the prosthesis sizes implanted and the surgical times.
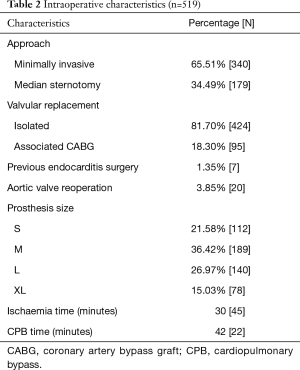
Full table
Any intraventricular conduction disorders presented by the patients prior to the operation were also collected (Figure 2).
The main inpatient postoperative complications and stay times in Intensive Care Units (ICU) and on the ward were collected (Table 3).
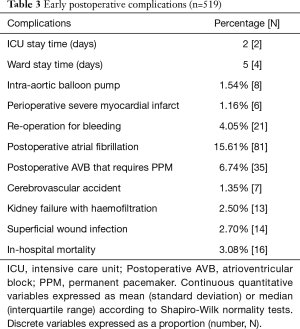
Full table
A univariate analysis was initially performed followed by a multivariate analysis, both reflected by their respective OR in Table 4. It was decided to use the model of all the possible equations. The maximum model set up presented an AIC of 265.9, a BIC of 342.4, a BIC and an AUC of 0.774 (0.04) with a confidence interval at 95% of 0.694 to 0.849. This presented a value >0.10 for the Hosmer-Lemeshow test. The final model presented as risk factors the technique used, height (cm), and the prior intraventricular conduction of the patient (Table 4). This presented an AIC of 250.1, a BIC 288.4 and an AUC 0.740 (0.04) with a confidence interval at 95% of 0.653 to 0.828. It presented a Hosmer-Lemeshow value of >0.10. A comparison was made of the predictive ability between the ROC curves of the maximum model and the final model. There were no significant differences (P=0.2661). We consider the predictive ability of this model to be good (5).
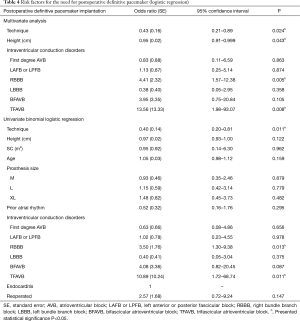
Full table
It was decided to descriptively study in our series the relationship between the proportion of postoperative AVB, height and implanted prosthesis size. Four groups were set up, divided according to the quartile corresponding to the height of the patient in our series and the proportion of postoperative AVB was recorded depending on the size of the prosthesis implanted in each of the groups Table 5.

Full table
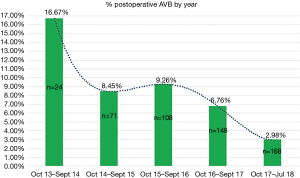
A graphical representation was performed of the cumulative incidence of postoperative AVB over time in our global series and in accordance with the technique used (Figures 3 and 4).
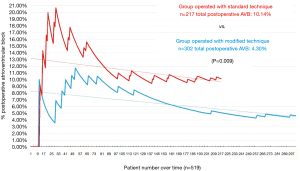
There were no significant differences (P>0.05) in the distribution of the size of implanted prosthesis nor in the intraventricular conduction disorders between groups operated with a different technique.
PPM dependency rate at 30 days from implant was 54.29%, at one year follow up, rate increases to 58.62% of dependency.
Discussion
The Perceval S prosthesis has been specially designed for implantation using minimally invasive approaches (6). For this reason, it was initially used as an alternative to conventional prostheses in high-risk patients, reporting promising results in terms of safety and efficacy. Their indications have increased, due in part to their excellent haemodynamic behaviour in small aortic roots (7). In this regard, considerable advantages have been reported in comparison to conventional aortic prostheses, other rapid-release aortic prostheses and even compared to transcatheter aortic valve implantation (TAVI) (7-9).
However, there is a great variability in the high rate of postoperative atrioventricular block in comparison to conventional prostheses, which requires the implantation of definitive pacemakers. This incidence, sometimes high, is described in the scientific literature to be between 8–23%, and raises doubts as to the benefits of implanting this type of prosthesis in medium-low risk patients, due to the increase in long-term morbidity and mortality that the pacemaker implantation entails (10). The radial force exerted by the stent against the aortic annulus appears to be the main reason for this high incidence. On the other hand, thermal sensing technology with Nitinol wire and the possibility of decalcifying the aortic annular ring seems to reduce this complication in this type of valves compared to TAVI (11).
As our group noted a high incidence of postoperative AVB in the initial phase of the programme, we decided to modify the implantation technique to reduce this complication. With our modified technique we reduced the overall rate of postoperative AVB to 4.30%. Firstly, the modification of the technique advocates a more thorough decalcification, which aims to avoid the impaction of calcium against the conduction system, similar to that which happens in TAVIs (12). Secondly, the repositioning of the prosthesis prior to the release, together with the reduction in ballooning time, allows the prosthesis to be expanded in the aortic annulus instead of being positioned below the valve plane and avoids the generation of a greater radial force on the membranous septum, factor that other groups have identified and therefore attempted to avoid by modifying the implantation technique. This reduction in ballooning time has no showed an increasement in paravalvular leaks in literature (13).
Finally, with an adequate measurement, it aims to prevent the oversizing of the native annular (4). The scientific evidence argues that oversizing generates a greater number of leaks, paravalvular leaks and an increase of paravalvular gradients due to the underexpansion of the Perceval S prosthesis (14,15). In our series, we did not observe significant differences between the rates of postoperative AVB in relation to age, isolated implanted prosthetic size or body surface area. However, we decided to analyse the patient prosthesis-height ratio since height is directly related to the size of the valve annulus; it has been reported that this can be a more accurate measurement than body surface area (16). We can observe a particular distribution in Table 5. In this we can see some really high incidence rates of postoperative AVB when we implant large sized (L or XL) prostheses in patients with low height, below the 50 percentile (occasionally above the 25–30%), which seems to be related to annular oversizing and is consistent with the height-postoperative AVB rate that seems to exist in our series (17).
In addition, our study sought to identify the risk factors that would predict the appearance of postoperative AVB after the AVR with Perceval prosthesis. Several groups have previously tried to identify these risk factors, both in conventional and non-sutured prostheses, always focusing on the technique and the preoperative characteristics of patients (18). Initially, we analysed the preoperative intraventricular conduction of our patients, observing that the carriers of right bundle branch block or trifascicular block, were significantly more susceptible to this complication. This is a fact previously described by other groups (19). It is possible that the anatomical relationship of the valve prosthesis with the membranous septum, where the shared portion of the left bundle of His is found, exerts a direct influence. This would explain why conduction would not be disordered in patients with preoperative LBBB, and electrical stimulation transmitted in a manner similar to pre-surgery. However, those patients with normal intraventricular conduction prior to the operation, had a greater frequency of postsurgical LBBB (20). Finally, we can observe how those patients with prior damage to the right conduction branch, such as those with RBBB or TFB, more easily developed postoperative AVB; this could be connected with the injury of the only branch of presurgical “healthy” conduction that they presented, in contrast to presurgical patients with normal intraventricular conduction or with prior LBBB.
We believe it is very important to make a brief comment on a recently published article, which we consider to be closely related to our research, the group of Vogt et al. recently published a series of 512 aortic valve replacements with perceval prostheses that analyzed the rate of postoperative AVB which precises PPM implant. His group advocates a technique with similarities to that described in this article, such as the importance of decalcification, deployment and placement of the prosthesis as well as the shorter time of ballooning. It stands out that, like in our work, the right bundle branch block and the technique performed seem to be risk factors at the time of suffering postoperative AVB. The added value of this study is the monitoring and long-term analysis of the pacemaker rythm of these patients. They analyze the risk factors that could influence pacemaker dependence, which markedly decreases over time. We consider it very interesting to carry out a similar analysis in the future in our series (21).
We consider the high cumulative incidence in the initial phase in both groups to be attributable to the learning curve of each implantation technique. The main variability between both rates of postoperative AVB incidence could be related to the two factors described above: intraventricular conduction disorders and annular oversizing. The latter could be corrected by modification of the implantation technique.
Limitations
The main limitation is that this is a non-randomised study. Complications and morbidity and mortality in the outpatient follow-up (except for the electrophysiological follow-up of the implanted pacemakers) were not analysed in this study. Not all patients presented an aortic root CT study before the operation; the aortic annulus was measured preoperatively primarily by trans-oesophageal echocardiography.
Conclusions
The implantation technique used, the RBBB and TFAVB prior to operation along with the height of the patient directly affect the rate of postoperative AVB, which requires the implantation of a PPM. The modified technique reduces the incidence of postoperative AVB by around 4.30% in our series. Controlling the risk factors could significantly reduce the incidence rate of postoperative AVB.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the local ethics committee. All patients recruited signed their informed consent.
References
- Mazine A, Bonneau C, Karangelis D, et al. Sutureless aortic valves: who is the right patient? Curr Opin Cardiol 2017;32:130-6. [Crossref] [PubMed]
- Miceli A, Santarpino G, Pfeiffer S, et al. Minimally invasive aortic valve replacement with Perceval S sutureless valve: early outcomes and one-year survival from two European centers. J Thorac Cardiovasc Surg 2014;148:2838-43. [Crossref] [PubMed]
- Bouhout I, Mazine A, Rivard L, et al. Conduction Disorders After Sutureless Aortic Valve Replacement. Ann Thorac Surg 2017;103:1254-60. [Crossref] [PubMed]
- González-Barbeito M, Pardo-Martinez P, Estévez-Cid F, et al. Prótesis sin sutura: ¿es posible reducir la tasa de bloqueos postoperatorios modificando la técnica de implante? Cirugía Cardiovasc 2018;25:257-62. [Crossref]
- Swets JA. Indices of discrimination or diagnostic accuracy: their ROCs and implied models. Psychol Bull 1986;99:100-17. [Crossref] [PubMed]
- Chauvette V, Mazine A, Bouchard D. Ten-year experience with the Perceval S sutureless prosthesis: lessons learned and future perspectives. J Vis Surg 2018;4:87. [Crossref] [PubMed]
- Liakopoulos OJ, Gerfer S, Weider S, et al. Direct Comparison of the Edwards Intuity Elite and Sorin Perceval S Rapid Deployment Aortic Valves. Ann Thorac Surg 2018;105:108-14. [Crossref] [PubMed]
- Meco M, Montisci A, Miceli A, et al. Sutureless Perceval Aortic Valve Versus Conventional Stented Bioprostheses: Meta-Analysis of Postoperative and Midterm Results in Isolated Aortic Valve Replacement. J Am Heart Assoc 2018;7. [Crossref] [PubMed]
- Wang N, Tsai YC, Niles N, et al. Transcatheter aortic valve implantation (TAVI) versus sutureless aortic valve replacement (SUAVR) for aortic stenosis: a systematic review and meta-analysis of matched studies. J Thorac Dis 2016;8:3283-93. [Crossref] [PubMed]
- Mehaffey JH, Haywood NS, Hawkins RB, et al. Need for Permanent Pacemaker after Surgical Aortic Valve Replacement Reduces Long-Term Survival. Ann Thorac Surg 2018;106:460-5. [Crossref] [PubMed]
- Hurley ET, O'Sullivan KE, Segurado R, et al. A Meta-Analysis Examining Differences in Short-Term Outcomes Between Sutureless and Conventional Aortic Valve Prostheses. Innovations (Phila) 2015;10:375-82. [Crossref] [PubMed]
- Pollari F, Grossmann I, Vogt F, et al. Risk factors for atrioventricular block after transcatheter aortic valve implantation: a single-centre analysis including assessment of aortic calcifications and follow-up. Europace 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Charles Blouin M, Bouhout I, Demers P, et al. Tackling the Issue of High Postoperative Pacemaker Implantation Rates in Sutureless Aortic Valve Replacement: Should Balloon Inflation be Removed from the Implantation Method of the Perceval Prosthesis? J Heart Valve Dis 2017;26:247-54. [PubMed]
- Baert J, Astarci P, Noirhomme P, et al. The risk of oversizing with sutureless bioprosthesis in small aortic annulus. J Thorac Cardiovasc Surg 2017;153:270-2. [Crossref] [PubMed]
- Cerillo AG, Amoretti F, Mariani M, et al. Increased Gradients After Aortic Valve Replacement With The Perceval Valve: The Role of Oversizing. Ann Thorac Surg 2018;106:121-8. [Crossref] [PubMed]
- Vasan RS, Larson MG, Levy D. Determinants of echocardiographic aortic root size. The Framingham Heart Study. Circulation 1995;91:734-40. [Crossref] [PubMed]
- Zhang R, Song Y, Zhou Y, et al. Comparison of aortic annulus diameter measurement between multi-detector computed tomography and echocardiography: a meta-analysis. PLoS One 2013;8:e58729. [Crossref] [PubMed]
- Nardi P, Pellegrino A, Scafuri A, et al. Permanent pacemaker implantation after isolated aortic valve replacement: incidence, risk factors and surgical technical aspects. J Cardiovasc Med (Hagerstown) 2010;11:14-9. [Crossref] [PubMed]
- Vogt F, Pfeiffer S, Dell'Aquila AM, et al. Sutureless aortic valve replacement with Perceval bioprosthesis: are there predicting factors for postoperative pacemaker implantation? Interact Cardiovasc Thorac Surg 2016;22:253-8. [Crossref] [PubMed]
- Toledano B, Bisbal F, Camara ML, et al. Incidence and predictors of new-onset atrioventricular block requiring pacemaker implantation after sutureless aortic valve replacement. Interact Cardiovasc Thorac Surg 2016;23:861-8. [Crossref] [PubMed]
- Vogt F, Moscarelli M, Nicoletti A, et al. Sutureless Aortic Valve and Pacemaker Rate: From Surgical Tricks to Clinical Outcomes. Ann Thorac Surg 2019;108:99-105. [Crossref] [PubMed]