The challenging management of hepatopulmonary fistulas
Introduction
Hepatopulmonary fistula represents a rare benign clinical entity related with a significant mortality risk. Interestingly the prevalence of the different etiological factors has changed over the years especially with the advance of liver ablating techniques and surgery. Although surgery remains the gold standard definite option, recent and less invasive techniques have given a new perspective in their management. A step by step approach to this entity, from diagnosis to treatment has to be reestablished in order to identify the role of interventional modalities and to develop a management algorithm.
Aetiopathogenesis
Hepatic hydatidosis although the most common predisposing factor for years, has been reported in only few case series in last three decades (1). Instead of it, other etiological factors have arisen related with the widespread use of conventional liver surgery and modern ablating techniques for liver diseases (2). All potential causes of hepatopulmonary fistulas can be summarised in the following five categories.
- Congenital;
- Hepatic hydatid disease or liver abscess (echinococcic, amoebic, pyogenic);
- Biliary tract obstruction secondary to tumors (more frequently biliary tree tumors);
- Blunt or penetrating injury (with or without an expanding hematoma causing obstruction);
- Iatrogenic fistulas (following liver resection, RFA ablation, radiation, thoracic drainage).
There are two major ways of fistula formation (3-5). The first follows the expanding mechanism of an infected biloma that resides underneath the diaphragm in a jaundiced patient. It is presumed that the existence of bile underneath diaphragm can erode tissues reaching the pleural space, bronchus or both (6,7). In addition, biliary stasis predisposes to abscess formation and further tissue damage. Factors contributing to biloma formation are diaphragmatic injuries with a concomitant liver trauma, tumors (most common cause according to literature), postoperative or post ablating biliary stenosis and lithiasis (3,8).
The other mechanism involves spreading of a hydatid liver cyst or other invasive liver process (e.g., amoebic abscess) to the adjacent lung or pleural space. In the same manner as infected biloma, the erosion of diaphragm gradually predispose to fistula formation (9). This inflammatory process usually is not associated with biliary obstruction in these patients, distinguishing them from the former group.
A fistula between right hemidiaphragm and nuda hepatis is the most frequently reported while a bronchobiliary fistula to the left lung has only been reported once in the literature (1).
Clinical features
Clinical features preceding the development of a hepatopulmonary fistula can be indicative of the underlying disease in the abdomen. Normally it is an evolving clinical situation with signs and symptoms of the abdominal infection, biliary obstruction and finally respiratory distress. Most frequent symptoms include fever, productive cough, chest pain, right upper abdominal pain, jaundice and bile stained sputum (2,10).
Biliptysis (bile stained sputum) and presence of bile in the pleural effusions, are both pathognomonic of the existence of a bronchobiliary fistula (1,11).
Sepsis or preliminary signs of the inflammatory process with tachypnea, tachycardia and low grade fever, or no specific symptoms (due to a chronic process) constitute the ongoing illness. The fulminant disease presents in the form of acute respiratory distress syndrome (ARDS) (12).
Another form of communication between abdomen and chest, the so called pleuro biliary fistula causes dry and irritating cough, chest pain and findings from the right chest, according to fluid accumulation in the right pleura and development of right basilar atelectasis. Pleuro biliary fistula is more difficult to be diagnosed unless high degree of suspicion exists, in a patient with predisposing factors.
Investigations
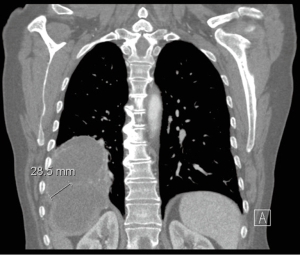
CT scan (Figure 1) remains a useful tool in delineating the pathology, especially when subtle symptoms exist. It can easily distinguish air into the biliary tree, an underlying hepatic abscess, effusion in the pleural space or basilar atelectasis and lung abscess (8). However in most of cases fails to demonstrate the fistulous tract.
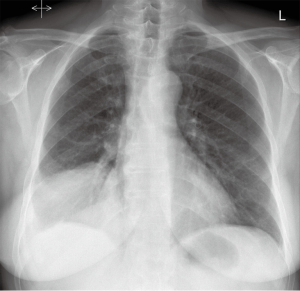
Some of these findings can also be suspected on plain chest (Figure 2), abdominal radiographs or demonstrated by ultrasonography.
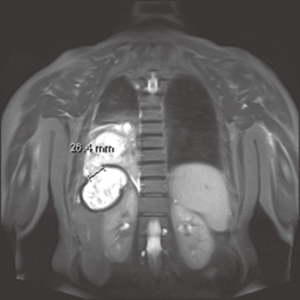
Bile stained sputum, bile presence in the pleural effusions or even jaundice should raise concerns for more precise depiction of the biliary tree. From the less invasive techniques, MRI (Figure 3) and MR cholangiography, can establish the diagnosis, although there are reports that it can also fail to demonstrate the fistulous tract (13). Hepatobiliary imino-diacetic acid (HIDA) scan can be considered, as a non-interventional alternative with increased diagnostic value, in patients with good performance status (14).
If further investigation is necessary, interventional depiction has to be considered. Cholangio-pancreatography (ERCP) or percutaneous transhepatic cholangiography (PTC) provide direct evidence of the fistula, possible distal biliary obstruction and offer therapeutic options (15,16). There are reports of spontaneously fistula closure after therapeutic PTC or ERCP (2).
Percutaneous tubography remains an immediate and absolute tool to delineate a hepatopulmonary fistula after evacuation of the abscess cavity in the chest or abdomen (2). It offers symptomatic relief to the patient and offers a surgical plan with minimal adverse effects, even under local anaesthesia.
Unstable septic patients in admission require circulatory and respiratory support followed by immediate surgical exploration.
Management
Regardless the interventional option applied, antibiotic therapy is primarily instituted to cover from gram negative microbes that usually are present into the sputum of these patients (17,18).
Of paramount importance is to maintain low pressures in the openings of fistula. This can be achieved with thoracostomy tube placement and biliary decompression with ERCP (endoscopic sphincterotomy and stend placement or nasobiliary drainage) or PTC (15,19). In presence of liver or lung abscesses it has to be drained under CT guidance in order to gain control of sepsis.
Literature review reveals cases of spontaneously closure of the fistula (up to 60% in posttraumatic fistulas) after endoscopic or subcutaneous biliary drainage (1). Other studies depict that the more conservative methods need a long standing drainage period that sometimes exceeds 5 weeks in duration. Reinsertion of tubes or drainage of new inflammatory foci gives a complete non-surgical approach in management of these patients. Nevertheless, the success of these methods depends on the degree of the inflammatory process.
In more chronic fistulas associated with clinical deterioration with respiratory compromise and uncontrolled abdominal and thoracic sepsis, aggressive therapy with surgery has considered traditionally the gold standard.
Surgical approach via thoracotomy can became in many cases the treatment option. Except drainage of pleural empyema, segmental excision of devitalized lung tissue or decortication, one can obtain immediate access to abdominal cavity through a severe diseased diaphragm, or take control over a penetrating injury with a phrenotomy. In addition, the hepatic area of involvement can be inspected for bilomas, abscesses and any other pathology that may require a formal access to abdomen. If there is no need for that, completion of therapy with biliary decompression can be managed postoperatively endoscopically. The principles according to Ferguson and Burford (20) for a successful management of the fistula involve:
- Early aggressive treatment by thoracotomy;
- Adequate subcostal drainage of the hepatic bed under direct vision;
- Secure closure of the diaphragmatic perforation by non-absorbable sutures;
- Decortication for the lung;
- Lobectomy for broncho biliary fistula and the awareness of the need for prophylactic decompression of the biliary tree.
These principles are applied to the most modern hybrid approaches (surgical plus endoscopic or interventional radiologic) that alleviate the risk of a major thoraco-abdominal operation.
The most challenging step of the operation is to restore the natural barrier between chest and abdomen. Diaphragm as part of the inflamed continuity among these compartments seems severely damaged as dissection proceeds. Huge defects can be managed with mobilisation of nearby tissue such as intercostal muscle or pericardial fat in a thoracic approach and with omentum majus in an abdominal approach (12,17). Synthetic mesh and AlloDerm® (17,21-23) have also been used in the past although the more desirable scenario is a primary closure with non-absorbable suture.
Reports of bronchoscopic attempts to seal the bronchobiliary communication and somatostatin analogues administration can all be considered, but evidence based data are limited.
Conclusions
Hepatopulmonary fistula although benign in nature carrying an unacceptable mortality risks up to 10.3% in some case series mainly due to surgical complications. From the first description by Ferguson and Burford in 1967 till present different approaches have been applied and with the introduction of less invasive techniques the results have significantly improved. Current data suggest a hybrid approach (surgical plus endoscopic or interventional radiologic) to this rare clinical entity individualised to each patient according to aetiology and severity of illness.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Liao GQ, Wang H, Zhu GY, et al. Management of acquired bronchobiliary fistula: A systematic literature review of 68 cases published in 30 years. World J Gastroenterol 2011;17:3842-9. [PubMed]
- Yoon DH, Shim JH, Lee WJ, et al. Percutaneous management of a bronchobiliary fistula after radiofrequency ablation in a patient with hepatocellular carcinoma. Korean J Radiol 2009;10:411-5. [PubMed]
- Warren KW, Christophi C, Armendariz R, et al. Surgical treatment of bronchobiliary fistulas. Surg Gynecol Obstet 1983;157:351-6. [PubMed]
- Gugenheim J, Ciardullo M, Traynor O, et al. Bronchobiliary fistulas in adults. Ann Surg 1988;207:90-4. [PubMed]
- Gries C, Branding G, Ritz JP, et al. Bronchobiliary fistula as a complication of Bülau drainage. Rofo 1998;169:315-7. [PubMed]
- Strange C, Allen ML, Freedland PN, et al. Biliopleural fistula as a complication of percutaneous biliary drainage: experimental evidence for pleural inflammation. Am Rev Respir Dis 1988;137:959-61. [PubMed]
- Porembka DT, Kier A, Sehlhorst S, et al. The pathophysiologic changes following bile aspiration in a porcine lung model. Chest 1993;104:919-24. [PubMed]
- D’Altorio RA, McAllister JD, Sestric GB, et al. Hepatopulmonary fistula: treatment with biliary metallic endoprosthesis. Am J Gastroenterol 1992;87:784-6. [PubMed]
- Johnson MM, Chin R Jr, Haponik EF. Thoracobiliary fistula. South Med J 1996;89:335-9. [PubMed]
- Kim YS, Rhim H, Sung JH, et al. Bronchobiliary fistula after radiofrequency thermal ablation of hepatic tumor. J Vasc Interv Radiol 2005;16:407-10. [PubMed]
- Singh B, Moodley J, Sheik-Gafoor MH, et al. Conservative management of thoracobiliary fistula. Ann Thorac Surg 2002;73:1088-91. [PubMed]
- Crnjac A, Pivec V, Ivanecz A. Thoracobiliary fistulas: literature review and a case report of fistula closure with omentum majus. Radiol Oncol 2013;47:77-85. [PubMed]
- Karabulut N, Cakmak V, Kiter G. Confident diagnosis of bronchobiliary fistula using contrast-enhanced magnetic resonance cholangiography. Korean J Radiol 2010;11:493-6. [PubMed]
- Annovazzi A, Viceconte G, Romano L, et al. Detection of a suspected bronchobiliary fistula by hepatobiliary scintigraphy. Ann Nucl Med 2008;22:641-3. [PubMed]
- Khandelwal M, Inverso N, Conter R, et al. Endoscopic management of a bronchobiliary fistula. J Clin Gastroenterol 1996;23:125-7. [PubMed]
- Deshmukh H, Prasad S, Patankar T, et al. Percutaneous management of a broncho-biliary fistula complicating ruptured amebic liver abscess. Am J Gastroenterol 1999;94:289-90. [PubMed]
- Chua HK, Allen MS, Deschamps C, et al. Bronchobiliary fistula: principles of management. Ann Thorac Surg 2000;70:1392-4. [PubMed]
- Rose DM, Rose AT, Chapman WC, et al. Management of bronchobiliary fistula as a late complication of hepatic resection. Am Surg 1998;64:873-6. [PubMed]
- Memis A, Oran I, Parildar M. Use of histoacryl and a covered nitinol stent to treat a bronchobiliary fistula. J Vasc Interv Radiol 2000;11:1337-40. [PubMed]
- Ferguson TB, Burford TH. Pleurobiliary and bronchobiliary fistulas. Surgical management. Arch Surg 1967;95:380-6. [PubMed]
- Gandhi N, Kent T, Kaban JM, et al. Bronchobiliary fistula after penetrating thoracoabdominal trauma: case report and literature review. J Trauma 2009;67:E143-5. [PubMed]
- Eryigit H, Oztas S, Urek S, et al. Management of acquired bronchobiliary fistula: 3 case reports and a literature review. J Cardiothorac Surg 2007;2:52. [PubMed]
- Rubikas R. Diaphragmatic injuries. Eur J Cardiothorac Surg 2001;20:53-7. [PubMed]