Comparison of Ivor Lewis esophagectomy and Sweet esophagectomy for the treatment of middle-lower esophageal squamous cell carcinoma
Introduction
Esophageal cancer is a leading cause of deaths due to cancer worldwide (1). Squamous cell carcinoma is the predominant histological subtype in Chinese patients (2). Although multidisciplinary treatments (MDTs) for esophageal cancer have made encouraging progress to improve survival, surgery is still an irreplaceable intervention among MDTs. Different surgical approaches, such as the Sweet esophagectomy, Ivor Lewis esophagectomy, McKeown esophagectomy, and transhiatal esophagectomy, have been applied clinically based on specific locations in the esophagus.
For cancer of the middle and/or lower segment of thoracic esophagus, the Ivor Lewis esophagectomy (3) and the Sweet esophagectomy via the left thoracic approach (4) are the mainstream standard surgical approaches in China currently (5). However, as no consensus has been reached about the choice of different thoracic entries in terms of recovery and survival, we designed this retrospective study to compare oncological benefits between the Ivor Lewis esophagectomy and Sweet esophagectomy.
Methods
As there was a remarkable technical evolution since 2009 in terms of surgical techniques, especially the spread of minimal invasive approach, our surgeons experienced a new learning curve. Since the change of surgical techniques may influence the outcome of our research, we analyzed our patients before 2010 who accepted open surgeries.
Inclusion and exclusion criteria
Patients admitted to the Beijing Cancer Hospital/Peking University Cancer Hospital for esophageal cancers who received inpatient surgery from August 2003 to December 2009 were eligible for this retrospective study. The surgeries of these patients were performed by the same chief surgeon. The institutional review board at the Peking University Cancer Hospital approved this retrospective study. The requirement of patient consent was waived because of the retrospective nature of the study.
Eligibility criteria included primary esophageal squamous cell carcinoma, tumor located in the middle or lower segment of the thoracic esophagus, no distant metastasis, no lymph node found to be >1 cm around the celiac trunk or in the neck before surgery on computed tomography (CT) or ultrasound examination, and the Ivor Lewis esophagectomy or Sweet esophagectomy determined suitable for the cure purpose. Exclusion criteria included adenocarcinoma, upper thoracic esophageal cancer, inoperable lesion even after neoadjuvant therapy, such as invading into an adjacent structure (the aorta, trachea, vertebrate, or heart), distant metastatic disease (including distant lymph node spreading), and use of other procedures, such as the McKeown procedure.
Propensity score matching
Propensity score matching was used to match cases in the Sweet and Ivor Lewis groups to create a “quasi-random experiment” from retrospective data (6,7). We utilized SPSS (version 22; IBM Corp., Armonk, NY USA) to calculate propensity scores by multiple variables including sex, age, tumor location, tumor differentiation (G status), and invasion (T status). Through a 1:1 matching algorithm and a caliper value of 0.2, we matched and analyzed 75 patients in the Sweet group and 75 patients in the Ivor Lewis group.
Preoperative diagnosis
Pathological diagnosis was made using gastrointestinal endoscopy. CT, routine air-contrast esophagography, and abdominal and supraclavicular ultrasound were performed to diagnose and eliminate metastatic lesions. Positron emission tomography/CT was considered only when distant metastasis was suspected. Pulmonary function testing and cardiac evaluation were performed as part of the preoperative assessment.
Surgical technique
Patients were treated with Sweet or Ivor Lewis esophagectomy during the investigation period according to tumor characteristics and surgeon’s preference. All patients underwent double lumen tracheal intubation and combined general and regional anesthesia with a thoracic epidural catheter.
Sweet esophagectomy (Sweet group) was defined as the partial esophagectomy and proximal gastrectomy with the removal of the abdominal and lower mediastinal lymph nodes. Patients were placed in the right lateral decubitus position. Thoracotomy was performed via the sixth or seventh intercostal space. Before dissection, resectability of the primary tumor was assessed. Dissection of the intrathoracic esophagus included en bloc resection of the esophageal cancer and the surrounding tissue. The proximal margin was at least 5 cm from the lesion, and frozen sections were performed as per routine to secure the tumor free margin. The distal edging was 5–8 cm from the lesion according to the tumor location. A subtotal gastric tube was then made using linear staplers through the diaphragm muscle incision, starting from the gastric fundus toward the lesser curvature. The extent of resection of the gastric fundus may vary due to the different status of tumor involvement. Celiac lymphadenectomy was performed through the diaphragm incision. The gastric tube was then pulled up with care to not twist the tube. An end-to-side gastroesophageal anastomosis was performed with a circular stapler, which passed through a small gastrotomy. A nasogastric and a nasoduodenal feeding tube were positioned separately to meet the purposes of gastric decompression and nutritional support. The gastrotomy was then closed by a linear stapler.
For the Ivor Lewis procedure (Ivor Lewis group), patients underwent a laparotomy followed by a thoracotomy. In brief, patients were placed in the supine position. The stomach was mobilized after initial abdominal exploration. A subtotal gastric tube was made using linear staplers as described above in the Sweet procedure. The gastric cardia was transected during dissection at this stage. Celiac lymphadenectomy was performed. After that, patients were placed in the left lateral decubitus position. The gastric tube was carefully pulled up to the chest cavity. An en bloc mobilization of the thoracic esophagus and mediastinal lymph node dissection were performed. Requirement for proximal margin was similar to the procedure via the left approach (the Sweet procedure). An end-to-side gastroesophageal anastomosis was performed with a circular stapler, and a nasogastric and a nasoduodenal feeding tube were positioned separated.
Pyloroplasty was not routinely performed in the two procedures. The anatomic position of all dissected lymph nodes was recorded.
TNM staging and lymph node stations were redefined according to the recommendations of the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC) through the 8th edition of the AJCC Cancer Staging Manual for esophageal carcinoma (8).
Perioperative management
The decision of extubation and ICU stay overnight was made based on physiological stability. On the 1st day after surgery, all patients were started on enteral feeding. On the 7th day after surgery, patients underwent esophagography. Once anastomotic leak was excluded, patients were started on food and water intake until full oral intake (although enteral feeding was stopped earlier in some patients due to intolerance, such as diarrhea, abdominal distention, or severe reflux). We remove the NG tube only when the patients have intake semiliquid for 1–2 days and no evidence of gastric retention existed.
Follow-up
Disease recurrence and survival were generally monitored at 1-month postoperatively and at 3-month intervals for 2 years, at 6-month intervals for the subsequent 3 years, and then at 1-year intervals. The follow-up was stopped once the patient achieved a 10-year survival. The last follow-up was performed in January 2018, and the overall survival (OS) was calculated from the date of surgery to the date of death or last follow-up.
Testing at follow-up included chest CT, ultrasound of the abdomen, neck, and supraclavicular area, complete blood cell count, and tests of hepatic and renal functions.
Statistical analysis
Perioperative outcomes, nutritional outcomes, and lymph node retrieval were compared between the two groups. Values were expressed as mean ± standard deviation. The Student’s t-test and Mann-Whitney U test were used for the analysis of normally and non-normally distributed data, respectively. The Pearson’s chi-squared (χ2) test was used to compare proportions (or Fisher’s exact test, as required). OS rates were estimated using the Kaplan-Meier method and compared using the log-rank test. P values for differences were calculated with a significance level of P<0.05. SPSS software was used for all analyses.
Results
During August 2003 to December 2009, 369 patients with esophageal squamous cell carcinoma were admitted to the Department of Thoracic Surgery, Ward II, for inpatient surgery. Among them, 262 patients with primary esophageal squamous cell carcinoma located in the middle and/or lower segment of the thoracic esophagus (location of the tumor was confirmed by surgeons during surgery) were treated during the investigation period. According to the inclusion and exclusion criteria described above, 170 patients were eligible for this research study. After propensity score matching, 75 patients in the Sweet group (from 76 patients) and 75 patients in the Ivor Lewis group (from 94 patients) were analyzed. After a median follow-up of 90.08 months (range, 3.87–172.43 months), 90 patients (60%) died. The 5-year OS rate for the whole group was 48.5%, whereas the 10-year OS was 35.6%.
General characteristics of patients in these two groups are summarized in Table 1. The two groups were equally matched in terms of sex, age, pathological staging, and the status of neoadjuvant therapy or adjuvant therapy.
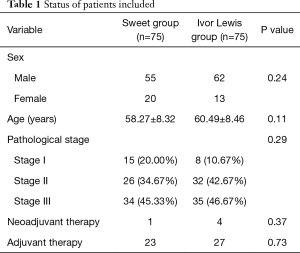
Full table
Postoperative characteristics and complications
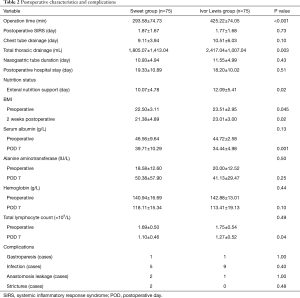
The postoperative characteristics and complications are summarized in Table 2. Compared to the left approach (Sweet) group, the Ivor Lewis group had a longer operation time (425.22±74.05 vs. 293.58±74.73 min, P<0.001) and a larger volume of total thoracic drainage (2,417.04±1,007.04 vs. 1,805.07±1,413.04 mL, P=0.003). However, no significant differences were found between the two groups in terms of the duration of SIRS, duration of chest tube drainage, length of postoperative hospital stay, and duration of postoperative nasogastric tube use. SIRS is defined as 2 or more of the following variables: (I) fever of more than 38 °C (100.4 °F) or less than 36 °C (96.8 °F); (II) heart rate of more than 90 beats per minutes; (III) respiratory rate of more than 20 breathes per minute or arterial carbon dioxide tension (PaCO2) of less than 32 mmHg; (IV) abnormal white blood cell count (>12,000/µL or <4,000/µL or >10% immature band forms).
Full table
Postoperative enteral feeding lasted longer in the Ivor Lewis group than in the Sweet group (12.09±5.41 vs. 10.07±4.78 d, P=0.02). Nutritional parameters, such as postoperative serum hemoglobin, total lymphocyte count, and plasma alanine aminotransferase level, at 7 days after surgery were identical between the two groups, except that the Ivor Lewis group had a significantly lower serum albumin level and the Sweet group had a lower lymphocyte count. No difference was found in body mass index (BMI) at 2 weeks after surgery between the two groups. These results indicate a similar impact on the metabolism and nutrition for these patients in different groups.
No difference was found between the two groups in the incidence of major complications, such as gastroparesis, anastomotic leak, or infection-related complications, after esophagectomy. Infection-related complications would include any infections after surgery (i.e., infection of the wound, lung, or thoracic cavity) and were diagnosed by bacterial culture of the thoracic or wound drainage, or sputum. Gastroparesis is defined as delayed emptying of the contrast agent in the esophagogram. Anastomotic leak is confirmed during the esophagogram when the contrast agent leaked into the mediastinum or thoracic cavity.
Lymph node dissection and survival
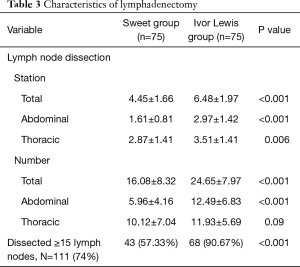
Compared to the left approach (Sweet) group, more lymph node stations were investigated and more lymph nodes were retrieved in the Ivor Lewis group, both in the thoracic cavity and in the abdomen (Table 3). In the Ivor Lewis group, 90.67% of the cases harvested more than 15 lymph nodes, but the ratio lowered to 57.33% in the Sweet group (P<0.001).
Full table
Detailed analysis of the lymph node dissection rate (= the number of patients whose certain lymph node station was dissected /the number of patients in the group) revealed that in the Ivor Lewis group, the dissection rate of No. 2+4, 8U, 16, 18, and 20 was higher and significantly different from that in the Sweet group (Table 4).
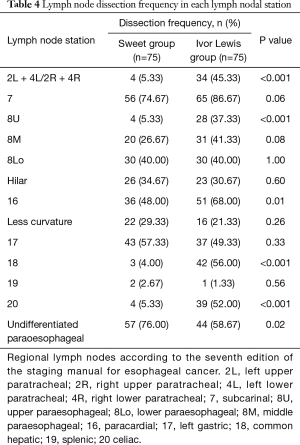
Full table
Concerning the N staging, although the lymph nodes dissected in the Sweet procedure were fewer in number than in the Ivor Lewis procedure, the distribution of patients with different N stages between both groups was similar (Table 5, P=0.65).
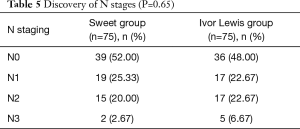
Full table
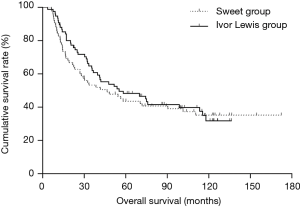
The Kaplan-Meier OS curves are depicted in Figure 1. The Sweet group had a similar outcome as the Ivor Lewis group. The 5- and 10-year OS rates were 43.6% and 35.1% in the Sweet group, respectively, and 48.1% and 31.7%, respectively, in the Ivor Lewis group (P=0.596, Figure 1).
Discussion
Sweet esophagectomy is traditionally the most frequently adopted procedure in China to treat patients with esophageal cancer located in the middle or lower segment of the thoracic esophagus (9). This procedure allows thoracic surgeons to explore the esophageal cancer at the beginning of surgery to confirm the surgical strategy. A single incision is the most obvious advantage of this procedure. However, this single incision needs to be large enough to achieve good exposure, thus potential postoperative pain, risk of incision infection, and possible coastal fracture could be prominent problems. It is also difficult for thoracic surgeons to dissect some stations of lymph nodes. According to 8th edition of the AJCC Cancer Staging Manual for esophageal carcinoma, the station 2+4 and 8U are locate behind the aorta and its branches through left thoracotomy. Dissection of these stations is very risky, if not impossible. Furthermore, in abdomen, dissection of station 18 and 20 are also difficult or risky because those areas are hard to be clearly exposed. Thus, for patients with suspicious lymph nodes in the above mentioned areas, R0 resection may not be easily achieved by Sweet esophagectomy.
Ivor Lewis esophagectomy is widely used and reported in the western countries. The most prominent advantage of this procedure, compared to the Sweet surgery, is a more thorough lymph node dissection in both the thoracic cavity and the abdomen, especially those locate in the upper mediastinum and around the celiac trunk and common hepatic artery. Furthermore, without aorta arch and descending aorta in the right thorax, the dissection of esophagus and anastomosis may be easier than that through Sweet procedure. For postoperative enteral nutrition support, the jejunostomy is easy to access during the abdominal part of Ivor Lewis esophagectomy. However, the operation time of Ivor Lewis esophagectomy may be longer than Sweet esophagectomy as two operations are includes in Ivor Lewis procedure. Postoperative pain also exists in this approach, and one more incision is needed. As Ivor Lewis esophagectomy treat abdomen first, it is not possible to explore the primary esophageal carcinoma first, although modern techniques (CT, ultrasonic endoscope) have developed to access the resectability of the primary tumor, careful preoperative evaluation is still essential to decide whether en bloc dissection will be achieved.
In developed countries, more than 50% of esophagectomies are currently performed using a minimally invasive approach. Minimal invasive Ivor Lewis esophagectomy is also more and more accepted by Chinese thoracic surgeons, especially those in the national or regional cancer centers. However, the analysis of open surgeries is still valuable for surgeons to keep improving the perioperative and long-term outcome of the future patients.
Lymph node dissection and survival
The fields and number of lymph nodes that need to be dissected remain controversial for patients with esophageal squamous carcinoma. Some Japanese researchers advocate for a three-field lymph node dissection (10), while other researchers consider two-field dissection as a standard procedure (11,12). Our research found that Ivor Lewis esophagectomy can achieve the standard two-field dissection, removing more stations (6.48±1.97 vs. 4.45±1.66) and numbers (24.65±7.97 vs. 16.08±8.32) of lymph nodes. More lymph nodes of No. 2+4, 8U, 18, and 20 were dissected from patients who underwent Ivor Lewis esophagectomy. The reason for these differences may that it is technically difficult to dissect these stations of lymph nodes. For the left (Sweet) approach, No. 2+4 and 8U are located behind the aorta and its branches, and No. 18 and 20 are rather deep through the diaphragm incision. If no suspicious lymph nodes exist, thoracic surgeons would more likely prefer to not risk bleeding to explore these underexposed areas through the Sweet esophagectomy incision,
The current National Comprehensive Cancer Network (NCCN) guidelines suggest a dissection of more than 15 lymph nodes for patients with esophageal cancer for an accurate staging. This study proved that it is easier to achieve this standard using Ivor Lewis esophagectomy (90.67% vs. 57.33%). Nevertheless, the N staging between the Ivor Lewis and Sweet groups showed no significant difference. As N staging was not matched during the propensity score matching, we consider this finding an indication that although the number of lymph nodes removed during the Ivor Lewis or Sweet esophagectomy is different, the efficiency of the N staging of both procedures is equivalent. Thoracic surgeons need not worry about N staging if they access all possible stations of lymph nodes even if they skip some technically difficult or risky stations.
Whether patients with esophageal cancer will benefit from more lymph node dissection is still debated. Although data from some studies (13-16) and our own center (17) agreed that the number of lymph nodes removed during esophagectomy was significantly associated with prognosis, some other studies indicate that extensive lymph node dissection is not associated with better prognosis (18,19). Furthermore, even for authors advocating for more lymph node dissections, the best cut-off remains unclear, with the recommended thresholds varying from 12 to 30 (20,21). In this study, despite more lymph nodes being removed in the Ivor Lewis group, no significant difference was found regarding survival between these two groups (P=0.596). The reason may either because not sufficient lymph nodes (e.g., ≥29) were removed from patients in the Ivor Lewis group to achieve a survival benefit or because sufficient lymph nodes were removed from patients in the Sweet group to achieve an accurate staging to guide the following treatment. Further randomized clinical research is needed to clarify this issue.
In 2015, Chinese researchers published a randomized prospective clinical trial on the comparison of the Ivor Lewis and Sweet procedures (5). To our knowledge, it was the first prospective clinical trial on this issue. Due to the characteristics of the surgery, it is impossible to be double-blinded. However, it was still a well-designed trial. The result of this clinical trial indicated a survival benefit of the Ivor Lewis procedure over the Sweet procedure, although the authors admitted that they had more experience with the Ivor Lewis procedure than with the Sweet procedure. This research also found that it was easier for surgeons to dissect more lymph nodes during the Ivor Lewis procedure; however, the authors did not analyze the influence of the number of removed lymph nodes on the survival of those patients.
In contrast, some other retrospective studies and one meta-analysis published around 2015 found no difference in survival between the Ivor Lewis and Sweet procedures (22-26). Our retrospective study agrees with this conclusion; however, our study still has some limitations. First, the number of the patients involved in this study was less. Second, before 2010, we adopted the Japanese lymph node station system of esophageal carcinoma, which is different from the current UICC/AJCC system. Applying a different classification system makes it difficult to accomplish a thorough assessment of lymph node dissection retrospectively.
Conclusions
Together, based on our research and the currently available published research, we believe it is difficult to conclude that one procedure is superior to the other. The Sweet and Ivor Lewis procedures are both safe and efficacious methods for the treatment of patients with middle or lower thoracic esophageal squamous carcinomas, and each has its own advantages and disadvantages. Thus, a change in the surgical incision alone may not be the best way to improve the survival of these patients. The choice of surgical procedure should depend on the preference of the thoracic surgeon to secure the safety of the operation. Physicians need to pursue the help of a multidisciplinary team and attempting at finding new and more efficacious treatments for these patients.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The institutional review board at the Peking University Cancer Hospital approved this retrospective study. The requirement of patient consent was waived because of the retrospective nature of the study.
References
- Thrift AP. The epidemic of oesophageal carcinoma: Where are we now? Cancer Epidemiol 2016;41:88-95. [Crossref] [PubMed]
- National Cancer Center, Disease Prevention and Control Bureau, Minister of Health. Chinese Cancer Registry Annual Report (In Chinese). Military Medical Science Press, 2012.
- Lewis I. The surgical treatment of carcinoma of the esophagus. With special reference to a new operation for growth of the middle third. Br J Surg 1946;34:18-31. [Crossref] [PubMed]
- Sweet RH. Transthoracic resection of the esophagus and stomach for carcinoma: analysis of postoperative complication, causes of death, and late results of operation. Ann Surg 1945;121:272-84. [Crossref] [PubMed]
- Li B, Xiang J, Zhang Y, et al. Comparison of Ivor- Lewis vs Sweet esophagectomy for esophageal squamous cell carcinoma: a randomized clinical trial. JAMA Surg 2015;150:292-8. [Crossref] [PubMed]
- Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011;10:150-61. [Crossref] [PubMed]
- Inacio MC, Chen Y, Paxton EW, et al. Statistics in Brief: An Introduction to the Use of Propensity Scores. Clin Orthop Relat Res 2015;473:2722-6. [Crossref] [PubMed]
- Rice TW, Ishwaran H, Ferguson MK, et al. Cancer of the Esophagus and Esophagogastric Junction: An Eighth Edition Staging Primer. J Thorac Oncol 2017;12:36-42.
- Chen SB, Weng HR, Wang G, et al. Surgical treatment for early esophageal squamous cell carcinoma. Asian Pac J Cancer Prev 2013;14:3825-30. [Crossref] [PubMed]
- Nishihira T, Hirayama K, Mori S. A prospective randomized trial of extended cervical and superior mediastinal lymphadenectomy for carcinoma of the thoracic esophagus. Am J Surg 1998;175:47-51. [Crossref] [PubMed]
- Swanson SJ, Batirel HF, Bueno R, et al. Transthoracic esophagectomy with radical mediastinal and abdominal lymph node dissection and cervical esophagogastrostomy for esophageal carcinoma. Ann Thorac Surg 2001;72:1918-24; discussion 1924-5.
- Visbal AL, Allen MS., Miller DL, et al. Ivor Lewie esophagectomy for esophageal cancer. Ann Thorac Surg 2001;71:1803-8. [Crossref] [PubMed]
- Mariette C, Piessen G, Briez N, et al. The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Ann Surg 2008;247:365-71. [Crossref] [PubMed]
- Groth SS, Virnig BA, Whitson BA, et al. Determination of the minimum number of lymph nodes to examine to maximize survival in patients with esophageal carcinoma: data from the surveillance epidemiology and end results database. J Thorac Cardiovasc Surg 2010;139:612-20. [Crossref] [PubMed]
- Peyre CG, Hagen JA, DeMeester SR, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg 2008;248:549-56. [PubMed]
- Morimoto J, Tanaka H, Ohira M, et al. The impact of the number of occult metastatic lymph nodes on postoperative relapse of resectable esophageal cancer. Dis Esophagus 2014;27:63-71. [Crossref] [PubMed]
- Yuan F, Qingfeng Z, Jia W, et al. Influence of Metastatic Status and Number of Removed Lymph Nodes on Survival of Patients with Squamous Esophageal Carcinoma. Medicine (Baltimore) 2015;94:e1973. [Crossref] [PubMed]
- van der Schaaf M, Johar A, Wijnhoven B, et al. Extent of lymph node removal during esophageal cancer surgery and survival. J Natl Cancer Inst 2015. [Crossref] [PubMed]
- Wong J, Weber J, Almhanna K, et al. Extent of lymphadenectomy does not predict survival in patients treated with primary esophagectomy. J Gastrointest Surg 2013;17:1562-8; discussion 1569. [Crossref] [PubMed]
- Altorki NK, Zhou XK, Stiles B, et al. Total number of resected lymph nodes predicts survival in esophageal cancer. Ann Surg 2008;248:221-6. [Crossref] [PubMed]
- Bollschweiler E, Baldus SE, Schroder W, et al. Staging of esophageal carcinoma: length of tumor and number of involved regional lymph nodes. Are these independent prognostic factors? J Surg Oncol 2006;94:355-63. [Crossref] [PubMed]
- Zhang H, Wang J, Wang W, et al. A meta-analysis of esophagectomy: the comparative study of Ivor-Lewis operation and Sweet operation. Zhonghua Wei Chang Wai Ke Za Zhi 2014;17:892-7. [PubMed]
- Ma J, Zhan C, Wang L, et al. The sweet approach is still worthwhile in modern esophagectomy. Ann Thorac Surg 2014;97:1728-33. [Crossref] [PubMed]
- Ma Q, Liu W, Long H, et al. Right versus left transthoracic approach for lymph node-negative esophageal squamous cell carcinoma. J Cardiothorac Surg 2015;10:123. [Crossref] [PubMed]
- Wang ZQ, Wang WP, Yuan Y, et al. Left thoracotomy for middle or lower thoracic esophageal carcinoma: still Sweet enough? J Thorac Dis 2016;8:3187-96. [Crossref] [PubMed]
- Yang X, Zhan C, Sun F, et al. Efficacy comparison of Sweet versus Ivor-Lewis esophagectomy in the treatment of middle-lower esophageal squamous cell carcinoma. Zhonghua Wei Chang Wai Ke Za Zhi 2016;19:979-84. [PubMed]