
Predicting spread through air spaces (STAS) preoperatively: can imaging help?
More than 80% of primary lung cancers are non-small cell lung carcinomas (NSCLCs), with adenocarcinoma the most common histologic subtype of NSCLC. Diagnosis of NSCLC is primarily made by histologic assessment, which also informs treatment selection (1). Lung adenocarcinoma is well-known to be histologically heterogenous, as reflected in the 4th edition of the World Health Organization classification, which recommends the use of histologic subtyping in 5% increments. The five major histologic subtypes are lepidic, acinar, papillary, micropapillary, and solid (1). Predominant subtype is predictive of clinical behavior: lepidic is regarded as low-risk, acinar and papillary as intermediate-risk, and micropapillary and solid as high-risk (2). Ground glass opacity (GGO) on computed tomography (CT) has been shown to be associated with lepidic growth and consolidation is associated with invasive growth.
Tumor spread through air spaces (STAS) is a newly identified morphologic feature of tumor invasion. Our group was the first to define STAS, which we described as the presence of tumor cells beyond the edge of the main tumor (1,3). STAS is an independent predictor of outcomes in squamous cell carcinoma (4,5) and pleomorphic carcinoma (6), as well as in lung adenocarcinoma. It is a significant risk factor for recurrence of small (≤2 cm) lung adenocarcinoma after limited resection (3). The 2015 World Health Organization classification introduced STAS as a new route of invasion and included it in the exclusion criteria for minimally invasive surgery for adenocarcinoma (1).
Warth et al. defined STAS as a detachment of small, solid cell nests (at least 5 tumor cells) away from the main tumor mass and classified it according to the distance from the main tumor mass: limited type (<3 alveolar layers) or extensive type (>3 alveolar layers) (7). The authors showed that presence of STAS, regardless of extent, was associated with reduced recurrence-free survival and overall survival among patients with resected adenocarcinoma of any stage. In the study from our group, 38% of patients with small lung adenocarcinoma had STAS. Even on multivariable analysis, presence of STAS remained independently associated with risk of recurrence after limited resection (hazard ratio, 3.08; P=0.014) (3). Following the publication of our findings in 2015, studies from multiple independent institutions in several countries have validated the prognostic importance of STAS in patients with lung adenocarcinoma (8).
This strong association between STAS and locoregional and distant recurrence following limited resection has prompted investigators to question whether STAS can be predicted preoperatively, thus ensuring the most appropriate type of resection is selected (2). To this end, a limited number of studies have investigated the association between various morphologic features and STAS-positive tumors (Table 1).
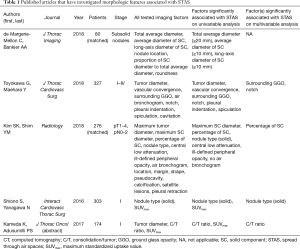
Full table
The feature most often studied for correlation with STAS is size. Toyokawa et al. and de Margerie-Mellon et al. observed that the total size of the lesion was significantly associated with STAS (9,10). The study by Toyokawa et al. included a wide cohort of adenocarcinomas, across all stages, and showed a significant difference between lesions >2 cm (54% of STAS-positive lesions) and ≤2 cm (46% of STAS-positive lesions) (P=0.02) (10). In the study by de Margerie-Mellon et al., which considered only subsolid nodules, lesions >2 cm were associated with STAS (9).
All of the studies that considered size or percentage of solid component (PSC) and consolidation/tumor (C/T) ratio, with the exception of the study from Shiono et al. (11), observed that presence of STAS increased as size of solid component or PSC increased (9,12-14). de Margerie-Mellon et al. observed that presence of solid component ≥10 mm in subsolid lesions was associated with STAS (9). Kim et al. observed that maximum diameter of solid component and PSC were associated with STAS. PSC remained associated with STAS on multivariable analysis and had a stronger discriminative power than maximum diameter of solid component: STAS was present in lesions with PSC ≥40% but not in lesions with ground glass component ≥60%. A PSC cutoff of 90% had discriminatory power for detection of STAS, with high sensitivity (13,15). Likewise, Toyokawa et al. demonstrated that higher C/T ratio was associated with STAS: 82% of STAS-positive lesions had a C/T ratio >0.51 (12).
When density was investigated without consideration of tumor size, purely solid nodules were associated with STAS. In the study by Shiono et al., 79% of STAS-positive lesions were solid, and 21% were nonsolid (P<0.001) (11). Similarly, in the study by Kim et al., 77% of STAS-positive lesions were solid, and 23% were part-solid (13). Toyokawa et al. demonstrated that absence of GGO was associated with STAS (12). In the study by Kim et al., no pure ground glass nodules were observed to be STAS positive; however, in other studies, 5%, 15%, and 11%, respectively, of pure ground glass nodules were observed to be STAS positive (9,12-14).
Other morphologic features, including margins, pleural indentation, tumor notch, cavitation, and presence of air bronchogram, among others, have been investigated. Toyokawa et al. and Kim et al. observed that presence of tumor notch, vascular convergence, central low attenuation of the lesion, and absence of air bronchogram were associated with STAS (12,13). Shiono et al. and Kameda et al. observed that higher maximum standardized uptake value (SUVmax) was associated with STAS (11,14).
On the basis of these results, it appears that pure-solid nodules, part-solid nodules with extensive solid component, and lesions with high SUVmax should be considered a priori positive for STAS and managed with lobectomy instead of limited resection. Pure ground glass nodules and subsolid nodules with predominant ground glass component were predominantly STAS negative and should therefore be considered for limited resection. Nevertheless, up to 15% of ground glass nodules were observed to be STAS positive. This led us to question whether presence or absence of STAS could be determined intraoperatively, to guide the extent of resection.
We recently determined that STAS is detectable on intraoperative frozen section, with high reproducibility (16). The estimated overall sensitivity and specificity across five pathologists, derived from the generalized estimating equation logistic regression model, were 71% [95% confidence interval (CI), 63–78%] and 92% (95% CI, 84–96%). The observed percent agreement was 75.4%. Once our results are validated by other investigators, the choice between limited resection and lobectomy could potentially be made intraoperatively on the basis of frozen section results.
Returning, then, to our original question: Can we predict STAS preoperatively on the basis of imaging features? At present, several morphologic features have been reported to be associated with STAS. However, the currently available data were derived from heterogeneous cohorts ranging from patients with all stages of adenocarcinoma to only patients with stage I disease. Future studies should be performed in larger cohorts to corroborate these findings. In addition, as STAS can be present in the peritumoral parenchyma, additional texture analysis studies are needed to characterize this area and determine whether it contains features that are predictive of STAS.
Acknowledgments
Funding: The authors’ laboratory work is supported by grants from the National Institutes of Health (P30 CA008748), the U.S. Department of Defense (LC160212), and the Emerson Collective Cancer Research Fund.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- World Health Organization Classification of Tumours of the Lung, Pleura, Thymus and Heart. Lyon, France: International Agency for Research on Cancer Press; 2015.
- Eguchi T, Kadota K, Park BJ, et al. The new IASLC-ATS-ERS lung adenocarcinoma classification: What the surgeon should know. Semin Thorac Cardiovasc Surg 2014;26:210-22. [Crossref] [PubMed]
- Kadota K, Nitadori J, Sima CS, et al. Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences after limited resection for small stage I lung adenocarcinomas. J Thorac Oncol 2015;10:806-14. [Crossref] [PubMed]
- Lu S, Tan KS, Kadota K, et al. Spread through air spaces (STAS) is an independent predictor of recurrence and lung cancer-specific death in squamous cell carcinoma. J Thorac Oncol 2017;12:223-34. [Crossref] [PubMed]
- Kadota K, Kushida Y, Katsuki N, et al. Tumor spread through air spaces is an independent predictor of recurrence-free survival in patients with resected lung squamous cell carcinoma. Am J Surg Pathol 2017;41:1077-86. [Crossref] [PubMed]
- Yokoyama S, Murakami T, Tao H, et al. Tumor spread through air spaces identifies a distinct subgroup with poor prognosis in surgically resected lung pleomorphic carcinoma. Chest 2018;154:838-47. [Crossref] [PubMed]
- Warth A, Muley T, Kossakowski CA, et al. Prognostic impact of intra-alveolar tumor spread in pulmonary adenocarcinoma. Am J Surg Pathol 2015;39:793-801. [Crossref] [PubMed]
- Ma K, Zhan C, Wang S, et al. Spread Through Air Spaces (STAS): A New Pathologic Morphology in Lung Cancer. Clin Lung Cancer 2019;20:e158-62. [Crossref] [PubMed]
- de Margerie-Mellon C, Onken A, Heidinger BH, et al. CT manifestations of tumor spread through airspaces in pulmonary adenocarcinomas presenting as subsolid nodules. J Thorac Imaging 2018;33:402-8. [PubMed]
- Toyokawa G, Yamada Y, Tagawa T, et al. Computed tomography features of resected lung adenocarcinomas with spread through air spaces. J Thorac Cardiovasc Surg 2018;156:1670-6.e4. [Crossref] [PubMed]
- Shiono S, Yanagawa N. Spread through air spaces is a predictive factor of recurrence and a prognostic factor in stage I lung adenocarcinoma. Interact Cardiovasc Thorac Surg 2016;23:567-72. [Crossref] [PubMed]
- Toyokawa G, Yamada Y, Tagawa T, et al. High frequency of spread through air spaces in resected small cell lung cancer. Anticancer Res 2018;38:1821-5. [PubMed]
- Kim SK, Kim TJ, Chung MJ, et al. Lung Adenocarcinoma: CT Features Associated with Spread through Air Spaces. Radiology 2018;289:831-40. [Crossref] [PubMed]
- Kameda K, Lu S, Eguchi T, et al. MA12.05 Can Tumor Spread through Air Spaces (STAS) in Lung Adenocarcinomas Be Predicted Pre- and Intraoperatively? J Thorac Oncol 2017;12:S4112.
- Naidich DP. Is spread of tumor through air spaces a concern for interpreting lung nodules on CT images? Radiology 2018;289:841-2. [Crossref] [PubMed]
- Eguchi T, Kameda K, Lu S, et al. Lobectomy is associated with better outcomes than sublobar resection in spread through air spaces (STAS)-positive T1 lung adenocarcinoma: A propensity score-matched analysis. J Thorac Oncol 2019;14:87-98. [Crossref] [PubMed]


