|
Original Article
Double CT imaging can measure the respiratory movement of small pulmonary tumors during stereotactic ablative radiotherapy
Ge Shen1#, Ying-Jie Wang2#, Hong-Guo Sheng1, Xiao-Ping Duan1, Jun-Liang Wang1, Wei-Jing Zhang1, Zhen-Shan Zhou1, Guang-Ying Zhu3, Ting-Yi Xia2
1Department of Radiation Oncology, Affiliated hospital of Academy of Military Medical Sciences; 2Army Radiation Cancer Center and Department of Radiation oncology, Air Force General Hospital; 3Department of Radiation Oncology, Beijing Cancer Hospital & Peking University Cancer Hospital, Beijing, China
Corresponding to: Ting-Yi Xia, MD. Army Radiation Cancer Center and Department of Radiation oncology, Air Force General Hospital. Beijing 100036, China. Email: 68434886@163.com.
Guang-Ying Zhu, MD. Department of Radiation Oncology, Beijing Cancer Hospital & Peking University Cancer Hospital, Beijing100036, China. Email: zgypu@yahoo.com.cn.
|
|
Abstract
Purpose: The purpose of this study was to investigate the application of double CT imaging to measuring the respiratory movement of small pulmonary tumors during stereotactic ablative radiotherapy (SABR).
Methods: A total of 122 small pulmonary tumors were measured in 45 patients. CT scans were conducted twice in all 122 tumors, once at the end of quiet inhalation and once at the end of exhalation. CT scans were conducted three times including at the end of quiet inhalation, at the end of exhalation and at free breathing in 36 tumors of 17 patients. The displacement of the tumor center in three directions was measured.
Results: The 3D motion of 122 tumors was 10.10±7.16 mm. On average, the tumors moved 1.96±2.03 mm (rang 0-9 mm) in the X direction, 5.19±4.69 mm (rang 0-19 mm) in the Y direction, and 7.38±6.48 mm (rang 0-26 mm) in the Z direction. The 3D motion of tumors in the lower lung (13.00±7.64 mm) was significantly greater than that in the upper lung (7.15±5.14 mm), P<0.01. The 3D motion of the lower left lung was 16.35±7.31 mm, which was significantly greater than that of the lower right lung (11.40±7.04 mm), P<0.05. Movement in the anterior lung in the Y direction was significantly larger than in the posterior lung. The motion was 7.49±5.43 mm and 4.04±3.82 mm respectively, P<0.01.
Conclusions: Double CT imaging provides accurate data for determining the outline of each target area during stereotactic ablative radiotherapy plane. The location of small pulmonary tumor foci was significantly affected by respiratory and cardiac motion.
Key words
Double CT scan; Small pulmonary tumors; Respiratory movement; Stereotactic ablative radiotherapy
J Thorac Dis 2012;4(2):131-140. DOI: 10.3978/j.issn.2072-1439.2012.01.04
|
|
Introduction
In recent years, the stereotactic ablative radiotherapy (SABR) or stereotactic body radiotherapy (SBRT) has been widely used in cancer treatment, which has achieved good results in patients with lung cancer ( 1-5). Senan et al. reviewed recent advances and controversies of SABR for stage I non-small cell lung cancer (NSCLC) ( 2). In order to avoid missing the target and overdosing surrounding critical structures, image guidance (particularly volumetric image guidance) for each treatment and motion management in cases with tumor motion greater than 1 cm are highly recommended ( 3). SABR allows treatment with increased irradiation doses to the site of the primary tumor by optimal lung sparing using modern radiotherapy technologies such as breathing motion compensation and image-guidance ( 4). Respiratory movement has been an important consideration for the delineating the targeted lung cancer area, especially in SABR treatment of small pulmonary tumors. The ICRU62 report separated the changes of the tumor target area due to the movement from planning target volume (PTV) and proposed the concept of internal target volume (ITV) ( 6). There are several ways to measure respiratory movement, including conebeam computed tomography (CBCT) ( 7, 8), electronic portal imaging device (EPID) ( 9), gold point tracking method ( 10, 11), dynamic nuclear magnetic resonance ( 12), six-CT scan ( 13), and 4D-CT method ( 14-16). Measurement of respiratory movement based on all of these methods has resulted in a similar
conclusion that within a given set of data from one patient, the
respiratory movement is larger in the lower lung than in the
upper lung. Heart movement also affects respiratory movement.
Among the methods mentioned above, some involve many steps,
are tedious to perform, and expose patients to high levels of
radiation ( 7, 8). Some methods are time-consuming ( 14-16). Other
method such as gold point tracking is an invasive procedure,
which has the risk of causing pneumothorax and is not suitable
for the elderly or patients with poor lung function ( 10, 11).
Based on the above consideration, the purpose of this study was
to investigate the application of double CT imaging to measuring
the respiratory movement of small pulmonary tumors during
SABR treatment, in order to find a convenient and simple way to
provide an accurate reference to delineate the target area. |
|
Materials and methods
Patients receiving SABR treatment for lung disease in our
institute between December 2009 and October 2010 were
included in the current study. The 4-slice spiral CT from Siemens
Company, Germany was used for scanning. During scanning,
the patient was in a supine position with both hands holding the
head. The body was fixed and scanned from the neck to the liver.
The layer thickness was 2.5 mm, and the scan time was 10-15
seconds. Patients were trained to do regular and quiet breathing
before CT scanning. Prior to scanning, each patient was
instructed through the microphone to maintain quiet breathing
and to hold their breath at the end of quiet inhalation and at
the end of exhalation so that CT scanning could be performed
during the inhalation and exhalation processes. Additionally, 17
patients received CT scanning during free breathing. CT images
were transferred to the planning system, and the corresponding
markers were calibrated. The positions of the tumor centers
were measured at the end of both inhalation and exhalation,
and during free breathing; the size of each tumor was measured
by CT at the end of quiet inhalation; and the target area was
outlined.
The center position of each tumor was measured at the end of
inhalation. fSI in the head-leg (superior-inferior, SI) Z direction
was defined as the ratio of the distance from the lung tip to the
tumor center to the lung height on the same side; the larger the
ratio, the closer the tumor to the bottom of the lung. A ratio
≤0.5 indicated the tumor was in the upper lung and >0.5 in the
lower lung; f LR in the left-right (LR) X direction was defined on the horizontal axis as the ratio of the distance from the upper
body midline to the tumor center to the distance from the body
midline to the inner thoracic wall; the larger the ratio, the closer
the tumor was to the lung edge. A ratio ≤0.5 indicated that the
tumor was closer to the midline and >0.5 closer to the left or
the right border of the lung. fAP in the anterior-posterior (AP)
Y direction was defined on the vertical axis as the ratio of the
distance from the anterior thoracic wall to the tumor center
to the distance from the anterior thoracic wall to the posterior
thoracic wall; the larger the ratio, the closer the tumor was to the
anterior thoracic wall. A ratio ≤0.5 corresponded to the anterior
portion of the lung, while a ratio >0.5 corresponded to the
posterior portion of lung. The free breathing position was defined
as the ratio of the total distanced moved during quiet inhalation
in the Z direction. A value of 0 indicated the same position as the
end of inhalation, 1 the same position as the end of exhalation,
and 0.5 the middle position between the end exhalation position
and the end inhalation position. The respiratory movement in
the X, Y, and Z directions, calculated as the 3D motion (total
movement) D2=X2+Y2+Z2.
|
|
Statistical Analysis
SPSS10.0 software was used for the statistical analysis. The
relationships between respiratory movement and the tumor
position, between respiratory movement and factors such as
tumor size, age, and gender, and between free breathing and the
end inhalation and exhalation positions were examined. The
t test was performed to compare the differences between the
groups. Multiple linear regressions were conducted to analyze
the impact of various factors on respiratory movement.
|
|
Results
General clinical data are shown in Table 1. Pathology, staging
and treatment are shown in Table 2. All of the tumors were lung
cancers or lung metastases. Nine cases were at stage I NSCLC, 1
case was at stage II who had not received medical treatment due
to old age, 1 case was at stage III who had not received medical
treatment due to myocardial infarction, and 2 cases had not
received medical treatment due to adenoid cystic carcinoma.
The other patients had received medical treatment before the
SABR treatment. Of the 45 patients, 24 patients had one tumor,
7 patients with two tumors, 2 patients with three tumors, 4
patients with four tumors, 2 patients with five tumors, 1 patient
with six tumors, 1 patient with seven tumors, 2 patients with
eight tumors, 1 patient with nine tumors, and 1 patient with
fourteen tumors. The range of the maximum tumor diameter
was 4.3-50.0 mm, the median was 10.1 mm, and the average was
16.90±12.90 mm; there were 60 tumors with diameters in the
range of 4.3-10 mm, 27 tumors in the range of 10.1-20 mm, 17 tumors in the range of 20.1-30 mm, and 18 tumors in the
range of 30.1-50 mm. The tumor volume ranged from 44.1 to
93, 744.5 mm 3. The median was 922.6 mm3. A total of 27 tumors
were located in the left upper lung, 20 were in the left lower lung,
34 were in the right upper lung, and 42 were in the right lower
lung. The respiratory movement for all 122 tumors in all directions
and the single-factor analysis of the total respiratory movement
are shown in Table 3. The tumors moved 1.96±2.03 mm (rang 0-9
mm) in the X direction, 5.19±4.69 mm (rang 0-19 mm) in the Y
direction, and 7.38±6.48 mm (rang 0-26 mm) in the Z direction.
The greater the position in the Z direction, the closer a tumor
was to the lower lung. The position in the Z direction was related
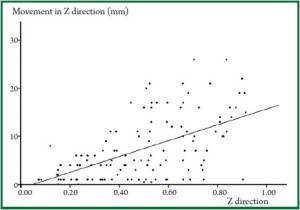
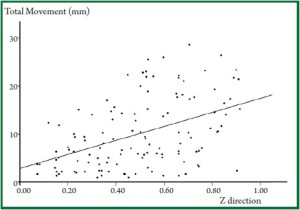
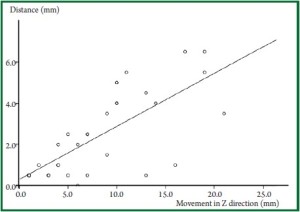
to the total movement in the Z direction ( Figure 1, Figure 2). Movement
and total movement in both left and right lower lungs in the Z
direction were significantly greater than the movement in both
upper lungs (P<0.001). The movement and total movement in
the left lower lung in the Y direction were significantly larger
than in the right lower lung, which may be related to the heart
beats (P<0.001 and P=0.017, respectively). Movement in the
anterior lung in the Y direction was significantly larger than in
the posterior lung (P<0.001). In addition, Y, Z, D movements
in patients under 65 years of age were significantly greater than
in patients over 65 years of age (P=0.015, P=0.035, P=0.017,
respectively); the movement in the X direction of tumors with
a diameter less than or equal to 20 mm was greater than tumors
larger than 20 mm in diameter (P=0.046). The largest respiratory movement of some tumors occurred in other directions though most tumors occurred in Z direction. For 20 out of 122 tumors, the largest respiratory movement
occurred in two or more directions. Of the remaining 102
tumors, 59.8% (61/102) underwent movement in the Z
direction 11.16±6.38 mm (rang 1-26 mm); 30.4% (31/102)
in the Y direction 8.58±5.42 mm (rang 2-19 mm); and 9.8%
(10/102) in the X direction 3.50±2.01 mm (rang 2-8 mm)
( Figure 3, Figure 4). The multi-factor analysis of all directions and the total
movement are shown in Table 4. Impact factors include age,
location (the right or the left side of the lower lung), tumor
size, and the Y and Z direction positions. There was no effect
of age on respiratory movement. Tumor size had an effect in
the movement in X direction (the smaller the tumor, the larger
the movement in X direction, P=0.015) but had no significant
effect on total movement (P=0.107); movement and total
movement of tumors in the left lower lung in Y direction were
significantly greater than in the right lower lung (P=0.021). The
position in the Y direction had a significant effect on Y direction
and total movement (P<0.001 and P=0.042, respectively). The position of Z direction had a significant effect on movement in
the Z direction and total movement (P<0.001 and P=0.017, respectively). The positions of 36 tumors were measured during free
breathing. The position and respiratory movement in the Z
direction during free breathing deviated from the axis by 0 to
6.5 mm. There were 24 tumors that moved from 0 to 2.5 mm, 6
from 3.5 to 4.5 mm, 6 from 5 to 6.5 mm. Overall, 94.4% (34/36)
of the tumors moved a distance ≤5.5 mm. Figure 5 shows the
relationship between a tumor’s distance from the center in the
Z direction and its respiratory movement during free breathing
(data from 12 tumors were the same, so 24 points are showed).
In 24 tumors with a small degree of movement, the respiratory
movement ranged from 1 to 16 mm, with a median of 4 mm
(mean±SD: 4.54±3.94 mm). In 12 tumors with a great degree
of movement, the respiratory movement ranged from 9 to 21 mm,
with a median of 12 mm (mean±SD: 13.58±4.32 mm). The offaxis
distance was significantly related to movement in the Z
direction (t=6.685, P<0.001). When respiratory movement was
1, the ratio of the positions of 36 tumors during free breathing
represented the relative position. A total of 36.1% (13/36) of
the tumors were close to the position of the end inhalation (a ratio of 0 to 0.2), 16.7% (6/36) close to the position of the end
inhalation (a ratio of 0.2 to 0.4), 11.1% (4/36) in between (a ratio of 0.4 to 0.6), and 27.8% (10/36) close to the position of
the end exhalation (a ratio of 0.8 to 1).
| Table 1. General description of the patients and tumors. |
| |
No. of cases (45) |
No. of Tumors (122) |
| Gender |
|
|
| Male |
29 |
77 |
| Female |
16 |
45 |
| Age |
|
|
| Median (range) |
59 (11-85) |
54 (11-85) |
| <65 |
30 |
99 |
| ≥65 |
15 |
23 |
| Table 2. The pathology, staging, and treatment of 45 patients. |
| Pathology |
TNM Stage |
No. of cases |
Without medical treatment |
One line cases |
Two lines cases |
Three lines and above |
| NSCLC |
1 |
9 |
9 |
- |
- |
- |
| |
IIB |
2 |
1* |
1 |
- |
- |
| |
IIIA |
1 |
1# |
- |
- |
- |
| |
IV |
10 |
- |
7 |
2 |
1 |
| SCLC |
Localized |
2 |
- |
2 |
- |
- |
| Colorectal cancer |
IV |
4 |
- |
1 |
1 |
2 |
| Hepatic carcinoma |
IV |
4 |
- |
2 |
2 |
- |
| Breast cancer |
IV |
2 |
- |
1 |
- |
1 |
| Adenoid cystic carcinoma |
IV |
2 |
2 |
- |
- |
- |
| Sarcoma |
IV |
2 |
- |
2 |
- |
- |
| Laryngeal |
IV |
1 |
- |
1 |
- |
- |
| Renal cancer |
IV |
1 |
- |
1 |
- |
- |
| Neurofibromatosis |
IV |
2 |
- |
2 |
- |
- |
| Endometrial cancer |
IV |
1 |
- |
1 |
- |
- |
| Bladder cancer |
IV |
1 |
- |
1 |
- |
- |
| Thyroid cancer |
IV |
1 |
- |
1 |
- |
- |
| NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; *81-year-old; #myocardial infarction. |
| Table 3. Average movement of 122 tumors [mean±standard deviation (mm)]. |
| Factor (no.) |
X |
T value |
P value |
Y |
T value |
P value |
Z |
T value |
P value |
D |
T value |
P value |
| All (122) |
1.96±2.03 |
|
|
5.19±4.69 |
|
|
7.38±6.48 |
|
|
10.10±7.16 |
|
|
| Lower lung (62) |
1.90±1.95 |
0.309 |
0.758 |
5.84±4.99 |
1.562 |
0.121 |
10.62±6.94 |
6.451 |
<0.001 |
13.00±7.64 |
4.952 |
<0.001 |
| Left lung (46) |
2.04±2.16 |
|
|
5.34±4.61 |
|
|
8.44±6.30 |
|
|
11.01±7.28 |
|
|
| Right lung (76) |
1.91±1.95 |
0.357 |
0.722 |
5.09±4.77 |
0.284 |
0.777 |
6.73±6.70 |
1.424 |
0.157 |
9.53±7.07 |
1.11 |
0.267 |
| Upper left (26) |
2.11±2.59 |
|
|
5.11±4.83 |
|
|
4.24±4.21 |
|
|
7.78±5.94 |
|
|
| Upper right (34) |
1.94±1.61 |
0.313 |
0.756 |
4.06±3.69 |
0.958 |
0.342 |
3.97±3.29 |
0.279 |
0.781 |
6.64±4.36 |
0.861 |
0.393 |
| Lower left (20) |
2.50±2.52 |
|
|
8.85±6.31 |
|
|
12.25±7.31 |
|
|
16.35±7.31 |
|
|
| Lower right (42) |
1.62±1.59 |
1.667 |
0.101 |
4.40±3.53 |
3.544 |
<0.001 |
9.85±6.76 |
1.272 |
0.208 |
11.40±7.04 |
2.461 |
0.017 |
| Anterior (40) |
2.22±2.10 |
|
|
7.49±5.43 |
|
|
7.07±5.46 |
|
|
11.32±6.80 |
|
|
| Posterior (82) |
1.83±1.99 |
1.007 |
0.316 |
4.04±3.82 |
4.085 |
<0.001 |
7.54±6.96 |
0.373 |
0.710 |
9.49±7.30 |
1.339 |
0.183 |
| Inside (47) |
1.72±1.81 |
|
|
4.30±4.14 |
|
|
7.18±7.02 |
|
|
9.31±7.49 |
|
|
| Outside (75) |
2.11±2.14 |
1.016 |
0.312 |
5.74±4.93 |
1.664 |
0.099 |
7.51±6.12 |
0.270 |
0.788 |
10.59±6.50 |
0.963 |
0.388 |
| Male (77) |
1.83±2.10 |
|
|
4.85±4.35 |
|
|
7.06±6.15 |
|
|
9.64±6.15 |
|
|
| Female (45) |
2.22±1.89 |
1.029 |
0.306 |
5.82±5.25 |
1.094 |
0.276 |
8.08±7.04 |
0.873 |
0.404 |
11.05±7.89 |
1.052 |
0.295 |
| <65 (99) |
1.97±2.12 |
|
|
5.71±4.87 |
|
|
8.03±6.87 |
|
|
10.90±7.58 |
|
|
| ≥65 (23) |
2.00±1.60 |
0.064 |
0.949 |
3.09±3.16 |
2.456 |
0.015 |
4.87±3.49 |
2.138 |
0.035 |
6.98±3.57 |
2.418 |
0.017 |
| ≤20 mm (87) |
2.21±2.21 |
|
|
5.49±5.05 |
|
|
7.80±6.81 |
|
|
10.77±7.51 |
|
|
| >20 mm (35) |
1.40±1.35 |
2.014 |
0.046 |
4.51±3.68 |
1.041 |
0.300 |
6.51±5.56 |
0.994 |
0.322 |
8.65±6.00 |
1.490 |
0.139 |
| Table 4. Multivariate analysis of all directions and total respiratory movement. |
| Factors |
X direction |
Y direction |
Z direction |
Total movement D |
| F |
Sig. |
F |
Sig. |
F |
Sig. |
F |
Sig. |
| Age |
0.013 |
0.908 |
0.714 |
0.400 |
1.993 |
0.161 |
1.883 |
0.173 |
| Diameter |
6.038 |
0.015 |
1.643 |
0.202 |
1.021 |
0.314 |
2.635 |
0.107 |
| Left and right lower lung |
1.850 |
0.176 |
5.438 |
0.021 |
1.538 |
0.217 |
4.048 |
0.047 |
| Y position |
1.143 |
0.287 |
18.856 |
<0.001 |
0.102 |
0.750 |
4.223 |
0.042 |
| Z position |
2.322 |
0.130 |
0.165 |
0.685 |
16.824 |
<0.001 |
5.896 |
0.017 |
|
|
Discussion
In 2003, Erridge et al. reported the measurements of 25 tumor
cases ( 9). The tumor sizes were unknown, the movement in X
direction was 7.3±2.7 mm, the movement in the Y direction
was 9.4±5.2 mm, and the movement in the Z direction was
12.5±7.3 mm. In 2007, Liu et al. reported measurements of
166 foci obtained using 4D-CT; the average movement in X
direction was 1.2 mm, the average movement in the Y direction
was 2.1 mm, and the average movement in the Z direction was
5.0 mm ( 14). Among these tumors, 79 had GTV >100 cm 3, and
those with larger diameters had smaller ranges of movement. In
2011, Dobashi et al. reported the results of 17 tumors obtained
using 4D-CT ( 15). According to these data, the movement
was 1.55±0.97 mm in the X direction, 2.44±1.04 mm in the Y
direction, and 7.94±6.67 mm in the Z direction. There were two
cases of T1 tumors (≤3 cm), eight cases of T2 tumors (3 cm<
diameter ≤5 cm), six cases of metastatic tumors of unspecified
size, and one case of a T3 tumor (diameter >5 cm). The present
study used double CT imaging to measure 122 tumors, and we
report movement of 1.96±2.03 mm in the X direction, 5.19±4.69
mm in the Y direction, and 7.38±6.48 mm in the Z direction. The
largest tumors in this study had diameters ≤5 cm, 104 tumors
had diameters ≤3 cm, and 18 had diameters ranging from 30.1 to
50 mm. The results of the current study are similar to Dobashi’s
report ( 15). In 2008, Michalski et al. reported the use of 4D-CT to
measure 23 lung tumors before and after treatment, some were
shrunk by more than 10%, but 3 movement values did not
change significantly ( 16). In the present study, tumors with small
diameters showed greater movement in the X direction, but the
absolute value was small, and there was no significant effect on
the target borderline; tumor size had no effect on movement in
the Y or Z direction or total movement, and movement may be
more related to the number of tumors. Respiratory movement of the upper lung was significantly
less than that of lower lung. Shimizu et al. ( 10) measuring 6
tumors in middle lung and 8 tumors in lower lung using gold
point tracking method; the average movement in Z direction
was 6.2 (2.4-11.3) mm and 9.1 (3.4-24.0) mm, respectively.
Seppenwoolde et al. ( 11) used the gold point tracking method
to measure 21 tumors and found that the movement of upper
lung in Z direction was 2±2 mm while that in the lower lung
was 12±6 mm, P=0.005. Plathow et al. ( 8) reported in 2004 on
6, 4, and 9 tumors in upper, middle, and lower lung, respectively.
During quiet breathing, the movement of the upper lung in 3
directions (X, Y, Z) was 3.4±1.6 mm, 2.8±1.3 mm, and 4.3±2.4 mm;
that in the middle lung was 4.3±2.4 mm, 4.3±2.2 mm, 7.2±1.8 mm;
and that in the lower lung was 6.0±2.8 mm, 6.1±3.3 mm, 9.5±4.9
mm. The movements of lower lung tumors in 3 directions were
significantly greater than the movements of upper lung tumors,
P<0.05. Likewise, the movements of middle lung tumors in the
Z and Y directions were significantly greater than the movements
of upper lung tumors, P<0.05. The movements of lower lung
tumors in the Z-direction were significantly greater than the
movements of tumors in the middle lung, P<0.05. van Sörnsen de Koste JR et al. ( 13) described the use of
CT scanning to measure the movement of 29 lung tumors.
Regardless of whether they were grouped by anatomical location
or by lobe, the movements of different groups of tumors in the Z
direction were not statistically different. The authors explained
the difference did not translate into a statistical significance due
to the small sample size ( 13). A report by Michalski et al. ( 16)
indicated that the average movement of the tumors in the upper
lung before treatment was 2.2±0.5 mm in the X direction, 4.5±2.8
mm in the Y direction, and 7.2±3.8 mm in the Z direction, while
movement in the lower lung was 3.3±1.9 mm in the X direction,
3.7±1.9 mm in the Y direction, and 10.8±9.4 mm in the Z
direction. Even without performing any statistical analysis, from
a numerical point of view, it is clear that lower lung movement
in the Z direction was significantly greater than that in the upper
lung movement. In the 122 tumors in this study, the movements
of the upper and lower lung in the X and Y directions were
similar, but the average movement in the Z direction in the
lower lung (10.62±6.94 mm) was significantly greater than the
movement in the upper lung (4.09±3.72 mm), P<0.0001. These
results are similar to most reports in the literature. In addition to the location of tumors in the lungs, other
factors that impact their respiratory movement include thoracic
breathing and cardiac motion. In this study, we found that
movement in the Y direction was related to the location of the
tumor on the Y axis, which may be related to thoracic breathing.
Total movement and movement in the Y direction of tumors in
the left lower lung were significantly greater than those of tumors
in the right lower lung, and this can be related to the heart beat.
The Y position had a significant effect on the total movement and movement in the Y direction, indicating that the closer the tumor
is to the anterior chest wall, the greater the movement; total
movement and movement in the Y direction for tumors in the
left lower lung were greater than in the right lower lung, which
demonstrates the effect of the heart beat on tumor movement.
Our data revealed that the maximum direction of the
movement was not in the Z direction for 40.2% of the patients,
suggesting that many patients may be accustomed to thoracic
breathing. Movement in Y direction was greater than in the
Z direction, as shown in Figure 1. In some patients, however,
movement in Y direction was smaller than in Z direction,
and in others, their actual movement was greater than the
conventionally estimated movement, as described in Figure 2.
Consistent with this, Erridge ( 9) reported that the maximum
movement in the Y direction was 34 mm, which was greater
than the movement in the Z direction (21 mm). In addition, the
movement of the heart has a significant effect on the movement
of tumors in the left lower lung tumor in the Y direction, and
this should be considered when delineating the ITV. Our results
indicate that respiratory movement is the lowest for tumors in
the lower lung and highest for tumors in the upper lung. Due to the specificity of the respiratory movement of each
tumor, each of the patients was asked to undergo respiratory
movement measurements to accurately outline the ITV
boundaries and to understand the movement of tumors in
different positions during quiet breathing. A larger deviation
occurs when only group data are used to mark the target area
boundaries.
There are many methods to determine the location of tumors
in the lung. Lung tumors were divided into groups according to
whether they occurred in the middle and lower lobes in several
reports ( 9-11). van Sörnsen de Koste ( 13) used X-ray films
to indicate the boundaries between the upper and lower, the
anterior and posterior, and the median and lateral portions of the
lung. Michalski ( 16) used the T5 vertebral body as the boundary
between the upper and lower, the anterior and posterior, and
the median and lateral halves of the lung. Plathow ( 12) used the
T3/4 vertebral space as the boundary between the upper and
middle lung, and T6/7 was used as the boundary between the
middle and lower lung. This study quantified the tumor position
based on the ratio of the three-dimensional coordinates of each
tumor. More accurate positioning allows for more detailed
analysis of the data. The general law of lung tumor movement can provide an
important practical reference ( 17, 18). It provides the basis for
the ITV boundaries. It can initially determine tumor movement
based on tumor position compare the estimated value according
to the tumor site and population data with the actual measured
value. We found one patient whose tumor movement in the Z
direction exceeded 40 mm, which is greater than the movement
observed during quiet breathing movement in all other patients. After repeated investigation, we found that the patient was taking
deep breaths during the CT scan. When we performed a new CT
scan during quiet breathing, we found that the tumor movement
in the Z direction was 20 mm. Therefore, it is important to
repeatedly remind the patient to breathe quietly and to avoid
deep breathing. Dobashi S. ( 15) used the bandage method to
reduce the breathing movement in young patients. This is an
effective method, but it may make elderly and ill persons or
persons with poor lung function feel sick. Currently, many scholars use free breathing in the SBRT
treatment positioning methods ( 20-22). This study found that
the tumor position was random during free breathing; only
11.1% of the tumors were in the same position at the end of
exhalation and the middle of inhalation. Our results indicate that
only using the data collected at the free breathing site will result
in errors in the ITV outline sometimes. However, expanding
the ITV range to avoid missing the target area will lead to
unnecessary damage to normal tissue. For large tumors, the loss
caused by these deviations may not be obvious. However, most
of the tumors treated by SBRT are small, and expanding the
outline will result in greater harm to normal tissue. There are many ways to measure respiratory movement. The
use of EPIs ( 9) or X-ray examination ( 19) is not as accurate
as CT or magnetic resonance imaging (several groups have
used fluoroscopy to image and calculate displacements of the
tumor during the respiratory cycle, but this technique is not
very accurate either). CBCT ( 7) can be performed in real time
after placement, and in essence, the measurement is carried out
using CT, but the image clarity is not sufficient. The gold point
tracking method is an invasive operation ( 10, 11). Measurement
using nuclear magnetic resonance is time-consuming ( 12).
With both fast and slow methods of CT ( 13), if the breathing
phase is ignored, the measured data may be less accurate. The
accuracy of 4D-CT is higher ( 14, 15), but the equipment is more
expensive and is not widely available. This method also requires
multiple scans, resulting in the potential exposure of patients to
more radiation. It is also more time-consuming and is difficult to
implement practically because of the heavy workload. Baba et al. ( 23) reported the use of methods similar to those
described in the present study, i.e., they scanned once during free
breathing and twice during inhalation and exhalation, with the
breath held in between. However, their paper did not illustrate
whether measurements were taken during quiet inhalation and
at the end of exhalation. In this study, CT images were collected
twice, once at the end of quiet inhalation and once at the end
of exhalation to obtain the range of movement. This is more
practical and provides accurate results, but it requires repeated
training of the patients by illustrating how to hold the breath
after quiet inhalation and at the end of exhalation. In conclusion, double CT imaging is a convenient, accurate,
and practical method for measuring the movement of lung tumors. This method should be investigated further in future
studies.
|
|
References
- Xia T, Li H, Sun Q, Wang Y, Fan N, Yu Y, et al. Promising clinical outcome
of stereotactic body radiation therapy for patients with inoperable Stage I/
II non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006;66:117-25.[LinkOut]
- Senan S, Palma DA, Lagerwaard FJ. Stereotactic ablative radiotherapy
for stage I NSCLC: Recent advances and controversies. J Thorac Dis
2011;3:189-96.[LinkOut]
- Chang JY. Stereotactic ablative radiotherapy for stage I NSCLC: Successes
and existing challenges. J Thorac Dis 2011;3:144-6.[LinkOut]
- Guckenberger M. What is the current status of Stereotactic body
radiotherapy for stage I non-small cell lung cancer? J Thorac Dis
2011;3:147-9.[LinkOut]
- Nagata Y, Wulf J, Lax I, Timmerman R, Zimmermann F, Stojkovski I, et al.
Stereotactic radiotherapy of primary lung cancer and other targets: results
of consultant meeting of the International Atomic Energy Agency. Int J
Radiat Oncol Biol Phys 2011;79:660-9.[LinkOut]
- ICRU report 62: Prescribing, recording and reporting photo beam therapy
(supplement to ICRU report 50). International Commission on Radiation
Units and Measurements, 1999. Available Online: http://en.wikibooks.
org/wiki/Radiation_Oncology/Physics/ICRU[LinkOut]
- Li W, Purdie TG, Taremi M, Fung S, Brade A, Cho BC, et al. Effect of
immobilization and performance status on intrafraction motion for
stereotactic lung radiotherapy: analysis of 133 patients. Int J Radiat Oncol
Biol Phys 2011;81:1568-75.[LinkOut]
- Bissonnette JP, Franks KN, Purdie TG, Moseley DJ, Sonke JJ, Jaffray DA,
et al. Quantifying interfraction and intrafraction tumor motion in lung
stereotactic body radiotherapy using respiration-correlated cone beam
computed tomography. Int J Radiat Oncol Biol Phys 2009;75:688-95.[LinkOut]
- Erridge SC, Seppenwoolde Y, Muller SH, van Herk M, De Jaeger K,
Belderbos JS, et al. Portal imaging to assess set-up errors, tumor motion
and tumor shrinkage during conformal radiotherapy of non-small cell lung
cancer. Radiother Oncol 2003;66:75-85.[LinkOut]
- Shimizu S, Shirato H, Kagei K, Nishioka T, Bo X, Dosaka-Akita H, et al.
Impact of respiratory movement on the computed tomographic images
of small lung tumors in three-dimensional (3D) radiotherapy. Int J Radiat
Oncol Biol Phys 2000;46:1127-33.[LinkOut]
- Seppenwoolde Y, Shirato H, Kitamura K, Shimizu S, van Herk M, Lebesque
JV, et al. Precise and real-time measurement of 3D tumor motion in lung
due to breathing and heartbeat, measured during radiotherapy. Int J Radiat
Oncol Biol Phys 2002;53:822-34.[LinkOut]
- Plathow C, Fink C, Ley S, Puderbach M, Eichinger M, Zuna I, et al.
Measurement of tumor diameter-dependent mobility of lung tumors by
dynamic MRI. Radiother Oncol 2004;73:349-54.[LinkOut]
- van Sörnsen de Koste JR, Lagerwaard FJ, Nijssen-Visser MR, Graveland
WJ, Senan S. Tumor location cannot predict the mobility of lung tumors:
a 3D analysis of data generated from multiple CT scans. Int J Radiat Oncol
Biol Phys 2003;56:348-54.[LinkOut]
- Liu HH, Balter P, Tutt T, Choi B, Zhang J, Wang C, et al. Assessing
respiration-induced tumor motion and internal target volume using fourdimensional
computed tomography for radiotherapy of lung cancer. Int J
Radiat Oncol Biol Phys 2007;68:531-40.[LinkOut]
- Dobashi S, Sugane T, Mori S, Asakura H, Yamamoto N, Kumagai M, et al.
Intrafractional respiratory motion for charged particle lung therapy with
immobilization assessed by four-dimensional computed tomography. J
Radiat Res (Tokyo) 2011;52:96-102.[LinkOut]
- Michalski D, Sontag M, Li F, de Andrade RS, Uslene I, Brandner ED,
et al. Four-dimensional computed tomography-based interfractional
reproducibility study of lung tumor intrafractional motion. Int J Radiat
Oncol Biol Phys 2008;71:714-24.[LinkOut]
- Giraud P, De Rycke Y, Dubray B, Helfre S, Voican D, Guo L, et al.
Conformal radiotherapy (CRT) planning for lung cancer: analysis of
intrathoracic organ motion during extreme phases of breathing. Int J Radiat
Oncol Biol Phys 2001;51:1081-92.[LinkOut]
- Rosenzweig KE, Hanley J, Mah D, Mageras G, Hunt M, Toner S, et al. The
deep inspiration breath-hold technique in the treatment of inoperable nonsmall-
cell lung cancer. Int J Radiat Oncol Biol Phys 2000;48:81-7.[LinkOut]
- Hof H, Herfarth KK, Münter M, Hoess A, Motsch J, Wannenmacher M,
et al. Stereotactic single-dose radiotherapy of stage I non-small-cell lung
cancer (NSCLC). Int J Radiat Oncol Biol Phys 2003;56:335-41.[LinkOut]
- Hamamoto Y, Kataoka M, Yamashita M, Shinkai T, Kubo Y, Sugawara Y, et
al. Local control of metastatic lung tumors treated with SBRT of 48 Gy in
four fractions: in comparison with primary lung cancer. Jpn J Clin Oncol
2010;40:125-9.[LinkOut]
- Gomez DR, Hunt MA, Jackson A, O'Meara WP, Bukanova EN, Zelefsky
MJ, et al. Low rate of thoracic toxicity in palliative paraspinal single-fraction
stereotactic body radiation therapy. Radiother Oncol 2009;93:414-8.[LinkOut]
- Wu J, Li H, Shekhar R, Suntharalingam M, D'Souza W. An evaluation of
planning techniques for stereotactic body radiation therapy in lung tumors.
Radiother Oncol 2008;87:35-43.[LinkOut]
- Baba F, Shibamoto Y, Ogino H, Murata R, Sugie C, Iwata H, et al. Clinical
outcomes of stereotactic body radiotherapy for stage I non-small cell
lung cancer using different doses depending on tumor size. Radiat Oncol
2010;5:81.[LinkOut]
Cite this article as: Shen G, Wang YJ, Sheng HG, Duan XP, Wang JL,
Zhang WJ, Zhou ZS, Zhu GY, Xia TY. Double CT imaging can measure
the respiratory movement of small pulmonary tumors during stereotactic
ablative radiotherapy. J Thorac Dis 2012;4(2):131-140. doi: 10.3978/
j.issn.2072-1439.2012.01.04
|