Pulmonary metastasectomy for sarcoma—survival and prognostic analysis
Introduction
Despite a lack of evidence, surgery has become the mainstay of treatment in patients with pulmonary metastatic sarcoma over the last decades. Five-year survival rates ranging from 15% to >50% following pulmonary metastasectomy (PM) in patients with resectable disease have been reported (1,2). Histology and grade of the primary tumour, number of metastatic nodules, disease-free interval (DFI) and resectability have been identified as prognostic survival factors in sarcoma (1-3). Recently, the association of systemic inflammation with poor prognosis in soft tissue sarcoma (STS) patients has been reported (4). Also, the nutritional patient status has been identified as a predictor of postoperative outcome and survival in various malignancies (5,6).
The aim of this retrospective single institution study was to analyse the postoperative survival and to assess the influence of selected factors related to primary tumour, lung metastases, associated surgical and oncological therapy as well as inflammatory parameters, inflammation scores and nutritional status, on the survival in patients undergoing PM for sarcoma.
Methods
All consecutive patients, who underwent curative intent PM for sarcoma at our single institution between January 2008 and December 2014 were retrospectively analysed. Selection criteria for PM were as follows: a stable primary tumour site, no evidence of synchronous extrapulmonary disease and intent of complete resection (R0) of pulmonary nodules. Cardiorespiratory fitness was routinely assessed in all surgical candidates. Before the surgery was offered, all patients were presented at the multidisciplinary tumor conference, where the treatment options were discussed. Resectability of lung metastases was assessed pre-operatively by computed tomography (CT). A muscle-sparing lateral thoracotomy enabling a thorough palpation of the lung was the preferred approach in majority of patients. Single peripheral metastases were resected by a video-assisted thoracic surgical (VATS) approach; however, the decision was based on the surgeon’s preference. Completeness of the resection was defined by pathological examination. Patients were followed-up with a CT scan 3 and 6 months after the first PM. If no recurrence occurred, follow-up intervals were prolonged to 6-month and then yearly controls.
The following variables were considered for statistical analysis: gender, age, histological subtype of sarcoma (STS vs. primary bone sarcoma), interval between surgery for primary tumor and PM, number and distribution of pulmonary metastases, administration of adjuvant therapy before and after PM, pre-PM C-reactive protein (CRP) serum level, neutrophil to lymphocyte ratio (NLR), modified Glasgow Prognostic Score (mGPS), prognostic nutritional index (PNI) and survival. Tumour histology was revised in accordance with the 4th edition of the “WHO Classification of Tumours of Soft Tissue and Bone” from 2013 (7). For the assessment of the systemic inflammatory markers, peripheral blood samples were collected on the day before PM or other invasive diagnostics during a routine preoperative check. Serum CRP levels were measured using a classical assay. The CRP value of ≤0.5 mg/dL was defined normal. The NLR calculated by dividing the absolute number of neutrophils by the absolute number of lymphocytes. The mGPS was calculated as follows: patients with both elevated CRP level (>0.5 mg/dL) and hypoalbuminaemia (<3.5 g/dL) were allocated a score of 2. Patients with either elevated CRP level or hypoalbuminaemia were allocated a score of 1, and patients with normal CRP level were allocated a score of 0. The association between the patient nutritional status and post-PM survival was assessed using the PNI calculated on the basis of preoperative data by the following formula: 10× serum albumin (g/dL) + 0.005× total lymphocyte count (cells/mm3).
Statistical analysis
Overall survival (OS) after PM was estimated by the Kaplan-Meier method using the date of the first PM as the starting point and the date of death or the most recent follow-up as the end point. Bilateral staged resections for synchronous metastases were counted as a single metastasectomy with the date of the first resection used for analysis. The interoperative interval was calculated as the interval between resection of the primary and date of PM. The variables were assessed using the univariable Cox proportional hazard model, giving data as hazard ratio (HR) with a 95% confidence interval (CI). The cut-off values of continuous variables were based on a receiver operating characteristic (ROC) analysis. The probability value (P) of less than 0.05 was considered statistically significant. The software used for statistical analysis was STATISTICA 10.0 (StatSoft, Tulsa, Oklahoma, USA).
Results
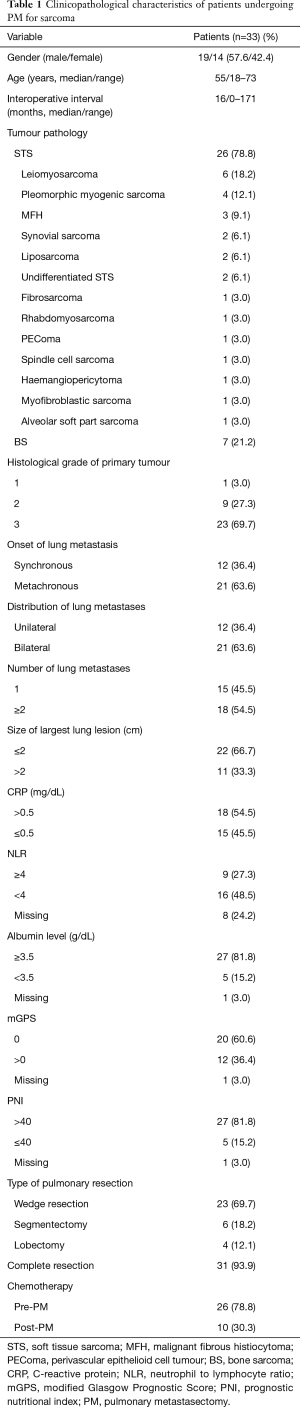
Between 2008 and 2014, 19 men and 14 women underwent pulmonary resections for metastatic sarcoma. The clinical characteristics of the 33 patients are listed in Table 1. The median age for the patients at the time of first PM was 55 years (range, 18–73 years). Bone sarcoma was the most common histological subtype (21.2%, n=7), followed by leiomyosarcoma (18.2%, n=6) and pleomorphic myogenic sarcoma (12.1%, n=4). The median interoperative interval between primary sarcoma resection and PM was 16 months (range, 0–171 months). Synchronous metastases with primary tumour were present in 12 (36.4%) patients. Nineteen (57.6%) patients had a perioperative interval longer than 1 year and 14 (42.4%) patients had a shorter perioperative interval (≤1 year).
Full table
The surgical approach consisted of muscle-sparing lateral thoracotomy (n=31, 93.9%) and VATS (n=2, 6.1%). At the time of the first pulmonary resection, the majority (23/33) of patients had a wedge resection, followed by anatomic segmentectomy in 6 patients, and lobectomy in 4 patients (left lower lobe n=2, right upper lobe n=1, and middle lobe n=1). Complete (R0) resection was accomplished in 31 (93.9%) patients. The majority of the patients had bilateral disease (n=21, 63.6%) and multiple pulmonary nodules (n=18, 54.5%). The maximum number of resected metastatic lesions in one patient was 22. No perioperative deaths occurred. Major (grade III/IV) complications occurred in 5 patients (15.2%), including 1 patient with postoperative haemothorax and 1 with a prolonged air leak requiring surgical intervention, 1 with pneumonia and 1 with postoperative phrenic nerve paresis. One patient required segmentectomy for lung infarction due to a pulmonary embolism on the 7th postoperative day. The average postoperative hospital stay was 6.6 days (range, 3.0–13.0 days).
A total of 26 patients (78.8%) had received chemotherapy before PM during the initial treatment of the primary tumour, 10 patients (30.3%) underwent chemotherapy as an adjuvant to PM.
The mean CRP of all analysed patients was 1.43 mg/dL (range, 0.03–11.50 mg/dL). The mean value of NLR was 3.63 (range, 0.40–8.65). The mGPS score was calculated in 32 patients. Twenty patients (60.6%) were allocated mGPS 0, 8 patients (24.2%) mGPS 1 and 4 patients (12.1%) mGPS 2. The mean PNI was 46.62.
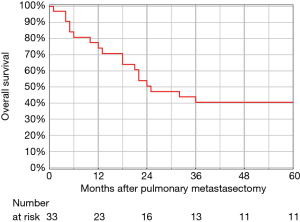
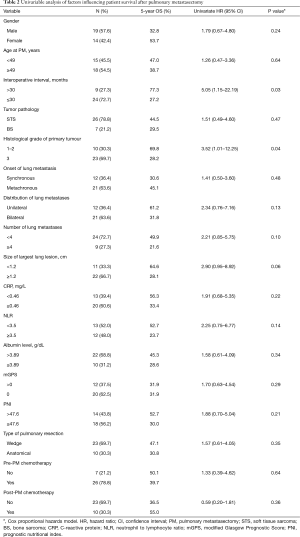
The 5-year OS rate after the first PM was 40.4% and was not influenced by age or gender (Figure 1). In the univariable analysis, 2 factors including perioperative interval and grade 3 of the primary tumour were identified as significant negative prognostic factors (Table 2). The ROC determined cut-off values for perioperative interval and grade of the primary tumour were dichotomized as follows: perioperative interval was 30 months [area under the curve (AUC) 0.67], and grade of the primary tumour was ≤2 (AUC 0.61). Due to the small number of events in this study, the multivariable analysis was not performed.
Full table
Discussion
Despite the absence of randomized data, the evidence supports PM for highly selected patients with metastatic sarcoma. Compared to other tumors, the patients with pulmonary metastatic bone sarcoma (BS) and STS have a worse prognosis following PM (8). The observed overall 5-year survival rate of 40.4% makes our cohort comparable with other published series. Histology of the primary tumor, DFI, size and number of lung lesions, unilateral disease, complete resection of metastases, and response to chemotherapy have been identified as favourable prognostic factors for pulmonary metastatic sarcoma.
According to a systematic review of 18 retrospective studies, including 1,196 patients who had PM for metastatic sarcoma between 1980 and 2006, performed by Treasure et al. survival after the first PM for BS was slightly better compared to STS, with 5-year OS rates of 34% vs. 25%, respectively (9). Lin et al. found even more favourable OS for BS vs. STS, with the 5-year OS rates of 53% vs. 37%, respectively, in a cohort of 155 patients with mixed sarcoma histology (10). In our series there was no significant survival difference between patients with BS and STS (5-year OS rate 29.5% vs. 44.5%; P=0.47). This may be the result of the small size of the analysed cohort. However, our finding is consistent with those previously reported by Reza et al. (11) and Mizuno et al. (2). The histological feature significantly associated with worse survival after PM in our sarcoma patient cohort was the grade of the primary sarcoma. Twenty-three patients with grade 3 sarcoma had shorter post-PM survival compared to those with lower grades (5-year OS rate 28.2% vs. 69.8%, respectively, P=0.04). Favourable survival in low grade sarcoma patients undergoing PM has also been reported (12).
A majority of authors describe prognostic value of DFI, defined as a period between curative resection of the primary tumour and the occurrence of metastasis, in patients with pulmonary metastatic sarcoma (2,8,10,12). A suggested cut-off associated with favourable survival range is from 12 to 36 months (13). According to Embún et al. the patients developing metastatic disease were not “disease-free” and the exact time point of recurrence or occurrence of metastasis cannot be accurately estimated (14). Based on this suggestion, in our analysis we refer to interoperative interval calculated between the resection of primary sarcoma and PM. The 5-year survival rate after PM in 9 (27.3%) of our patients with an interoperative interval longer than 30 months was significantly higher compared to 24 (72.7%) patients with a shorter intraoperative interval (77.3% vs. 27.2%, respectively, P=0.03). The post-PM survival of 12 (36.4%) patients with synchronous metastases did not significantly differ from that of 21 (63.6%) patients with metachronous metastases (5-year OS rate 30.6% vs. 45.1%, respectively, P=0.48). A similar finding was reported by Buddingh et al. (15).
There is no consensus on the maximum number, distribution and size of pulmonary lesions relevant in patients with lung metastasis from sarcoma being considered for PM. The number of resected metastases varying from 1 to <4 has been identified of prognostic value in several studies (2,16,17). Favourable prognosis in patients with unilateral metastatic lung disease has been reported by Kim et al. and Cariboni et al. in cohorts with mixed histology sarcoma (16,18). Also, the influence of the size of the largest pulmonary nodule on post-PM survival was noted (19,20). In our group none of these factors showed any significant correlation with OS. Similar observations on larger cohorts have been shared by other authors (21).
The feasibility of a complete resection was our main criterion in patient selection for PM. Due to peripheral or subpleural location of many sarcoma metastases, the majority of our patients underwent wedge resections. For centrally located or multiple nodules anatomical resections were performed. Our preferred approach was a muscle-sparing lateral thoracotomy that enabled us to bimanually palpate the lung parenchyma. In two patients with single peripheral lesions we performed VATS wedge resections. Two (6.1%) patients with the highest number of nodules underwent incomplete (R1) resection. In one patient with 22 small lesions of metastatic spindle cell sarcoma 1 of the wedge-resected metastases was necrotic and its negative margin was doubtful. Second R1 resection was due to 2 mm subpleural nodule incidentally found during pathological examination of a wedge with completely resected larger metastasis of synovial sarcoma. This small lesion was not digitally palpable during open surgery. Due to the small number of patients with incompletely resected metastases the comparison of OS between R0 and R1 resection was not performed. However, most of the published literature confirms the completeness of resection as a crucial prognosticator for OS. The cut-off value of the resection margin for PM in sarcoma has not been established. Welter et al. demonstrated that even laser metastasectomy with the closest resection margins did not result in higher recurrence rates (22).
Few studies analysed the prognostic role of systemic inflammation in metastatic lung disease. A favourable 5-year survival rate of 51% in 88 patients undergoing PM for sarcoma with low preoperative and day 3 postoperative level of CRP was recently reported by Pastorino et al. In the analysed cohort the 5-year survival of 34% in 188 patients with high CRP level was calculated (23). Ghanim et al. found that high fibrinogen, elevated CRP and the mGPS >0 were associated with poor OS in patients with pulmonary metastatic colorectal cancer (24). In our group preoperative CRP level, NLR and mGPS had no prognostic value. We observed that 12 of our patients with NLR ≥3.5 had worse survival compared to 13 patients with NLR<3.5, however, the difference was statistically insignificant (5-year survival rate 23.7% vs. 52.7%, respectively; P=0.14). Also, PNI was not demonstrated as a prognosticator in our small patient population. Eighteen (56.2%) patients with PNI ≤47.6 had a 5-year OS rate of 30% compared to 52.7% 5-year OS rate in 14 (43.8%) patients with higher PNI (P=0.21).
There is no consensus on the role of chemotherapy for metastatic sarcoma and to date its effectiveness as an adjunct to the surgery has not been proven (13). Due to the heterogeneous nature of sarcoma with many pathological subtypes of different aggressiveness, many treatment protocols exist. At our centre the sarcoma patient selection for a particular therapy concept (chemotherapy vs. surgery vs. multimodal) takes place within a sarcoma multidisciplinary team meeting. In this presented group there was no influence of either preoperative or postoperative chemotherapy on patient survival after PM.
Our study has several limitations: (I) due to the small size of the presented cohort, we analysed the patients with BS and STS collectively; (II) the single-centre retrospective design; (III) postoperative outcomes and survival rates were evaluated in patients highly selected for a curative intent surgery; (IV) there was no control group that would include metastatic sarcoma patients managed non-operatively with which to compare outcomes.
Conclusions
Our retrospective survival analysis revealed that pulmonary metastatic sarcoma patients with shorter interoperative interval and a higher tumour grade are likely to survive a shorter time after PM. Intensive follow-up after resection of the primary sarcoma and careful patient selection within the multidisciplinary team is highly significant. For objective assessment of the thoracic surgical treatment of metastatic sarcoma prospectively randomized control studies performed in high volume sarcoma centres are required.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was discussed with the trust’s Institutional Review Board (registration number 91_19 Bc). Individual patient consent for this retrospective observational study was waived.
References
- Smith R, Pak Y, Kraybill W, et al. Factors associated with actual long-term survival following soft tissue sarcoma pulmonary metastasectomy. Eur J Surg Oncol 2009;35:356-61. [Crossref] [PubMed]
- Mizuno T, Taniguchi T, Ishikawa Y, et al. Pulmonary metastasectomy for osteogenic and soft tissue sarcoma: who really benefits from surgical treatment? Eur J Cardiothorac Surg 2013;43:795-9. [Crossref] [PubMed]
- Chudgar NP, Brennan MF, Munhoz RR, et al. Pulmonary metastasectomy with therapeutic intent for soft-tissue sarcoma. J Thorac Cardiovasc Surg 2017;154:319-330.e1. [Crossref] [PubMed]
- Choi ES, Kim HS, Han I. Elevated preoperative systemic inflammatory markers predict poor outcome in localized soft tissue sarcoma. Ann Surg Oncol 2014;21:778-85. [Crossref] [PubMed]
- Sun J, Mei Y, Zhu Q, et al. Relationship of prognostic nutritional index with prognosis of gastrointestinal stromal tumors. J Cancer 2019;10:2679-86. [Crossref] [PubMed]
- Okada S, Shimada J, Kato D, et al. Clinical significance of prognostic nutritional index after surgical treatment in lung cancer. Ann Thorac Surg 2017;104:296-302. [Crossref] [PubMed]
- Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology 2014;46:95-104. [Crossref] [PubMed]
- Saleh W, AlShammari A, Sarraj J, et al. Surgical treatment of pulmonary metastasis: report from a tertiary care center. Asian Cardiovasc Thorac Ann 2018;26:296-301. [Crossref] [PubMed]
- Treasure T, Fiorentino F, Scarci M, et al. Pulmonary metastasectomy for sarcoma: a systematic review of reported outcomes in the context of Thames Cancer Registry data. BMJ Open 2012;2:e001736. [Crossref] [PubMed]
- Lin AY, Kotova S, Yanagawa J, et al. Risk stratification of patients undergoing pulmonary metastasectomy for soft tissue and bone sarcomas. J Thorac Cardiovasc Surg 2015;149:85-92. [Crossref] [PubMed]
- Reza J, Sammann A, Jin C, et al. Aggressive and minimally invasive surgery for pulmonary metastasis of sarcoma. J Thorac Cardiovasc Surg 2014;147:1193-200. [Crossref] [PubMed]
- Giuliano K, Sachs T, Montgomery E, et al. Survival following lung metastasectomy in soft tissue sarcomas. Thorac Cardiovasc Surg 2016;64:150-8. [Crossref] [PubMed]
- Marulli G, Mammana M, Comacchio G, et al. Survival and prognostic factors following pulmonary metastasectomy for sarcoma. J Thorac Dis 2017;9:S1305-15. [Crossref] [PubMed]
- Embún R, Fiorentino F, Treasure T, et al. Pulmonary metastasectomy in colorectal cancer: a prospective study of demography and clinical characteristics of 543 patients in the Spanish colorectal metastasectomy registry (GECMP-CCR). BMJ Open 2013;3:e002787. [Crossref] [PubMed]
- Buddingh EP, Anninga JK, Versteegh MI, et al. Prognostic factors in pulmonary metastasized high-grade osteosarcoma. Pediatr Blood Cancer 2010;54:216-21. [PubMed]
- Cariboni U, De Sanctis R, Giaretta M, et al. Survival Outcome and Prognostic Factors after Pulmonary Metastasectomy in Sarcoma Patients: A 18-Year Experience at a Single High-volume Referral Center. Am J Clin Oncol 2019;42:6-11. [Crossref] [PubMed]
- Sardenberg RA, Figueiredo LP, Haddad FJ, et al. Pulmonary metastasectomy from soft tissue sarcomas. Clinics (Sao Paulo) 2010;65:871-6. [Crossref] [PubMed]
- Kim S, Ott HC, Wright CD, et al. Pulmonary resection of metastatic sarcoma: prognostic factors associated with improved outcomes. Ann Thorac Surg 2011;92:1780-6. [Crossref] [PubMed]
- Weiser MR, Downey RJ, Leung DH, et al. Repeat resection of pulmonary metastases in patients with soft-tissue sarcoma. J Am Coll Surg 2000;191:184-90. [Crossref] [PubMed]
- Welter S, Grabellus F, Bauer S. Growth patterns of lung metastases from sarcoma: prognostic and surgical implications from histology. Interact Cardiovasc Thorac Surg 2012;15:612-7. [Crossref] [PubMed]
- Rehders A, Hosch SB, Scheunemann P, et al. Benefit of surgical treatment of lung metastasis in soft tissue sarcoma. Arch Surg 2007;142:70-5. [Crossref] [PubMed]
- Welter S, Arfanis E, Christoph D, et al. Growth patterns of pulmonary metastases: should we adjust resection techniques to primary histology and size? Eur J Cardiothorac Surg 2017;52:39-46. [Crossref] [PubMed]
- Pastorino U, Morelli D, Leuzzi G, et al. Baseline and Postoperative C-reactive Protein Levels Predict Long-Term Survival After Lung Metastasectomy. Ann Surg Oncol 2019;26:869-75. [Crossref] [PubMed]
- Ghanim B, Schweiger T, Jedamzik J, et al. Elevated inflammatory parameters and inflammation scores are associated with poor prognosis in patients undergoing pulmonary metastasectomy for colorectal cancer. Interact Cardiovasc Thorac Surg 2015;21:616-23. [Crossref] [PubMed]