
Mitral valve restenosis after closed mitral commissurotomy: case discussion
Introduction
At the beginning of heart valve surgery, closed mitral commissurotomy (CMC) and balloon dilatation were widely applied, but they were gradually replaced by mitral valvuloplasty or replacement due to their poor long-term effectiveness (1). Valve calcification/contracture and restenosis often occur after CMC or balloon dilatation, along with the lesions in the untreated valves and cardiac insufficiency (2). A re-operation is often required. With the development of bioprosthetic valve replacement and valvuloplasty, re-operation of valves in China is gradually increasing (3). Whether an open surgery or a thoracoscopic surgery should be performed remains controversial. In this manuscript, we present a case of mitral restenosis and calcification 30 years after CMC. The invited multidisciplinary experts gave detailed suggestions on intra- and post-operative monitoring and treatment.
Case presentation
The patient was a 56-year-old female who had experienced chest tightness, shortness of breath, palpitations, and fatigue for more than 10 years. She had received irregular diuretic medications before. Four days before her admission, the above symptoms worsened after a cold. She suffered from chest tightness and palpitations after mild exercise, along with cough and production of yellow (and sometimes pink) sputum, and she had no paroxysmal nocturnal dyspnea. She was diagnosed with “pulmonary infection and cardiac insufficiency” in a local hospital. Then she was referred to our center for further treatment.
Thirty years ago, she underwent a CMC procedure for rheumatic heart disease and mitral stenosis. She had a previous history of celecoxib allergy. She denied having any history of hypertension, coronary heart disease, diabetes, tuberculosis, or another related medical history. Also, she had no history of smoking or drinking. Physical examination showed that body temperature (T) was 37.4 °C; heart rate (HR) 80 beats/min; blood pressure (BP) 131/95 mmHg, respiratory rate (R) 19 breaths/min, body height 163 cm, and body weight 76 kg. She had no cyanosis. The heart rhythm was irregular, with grade 2/6 apical diastolic murmurs, which were conducted to the left axillary midline. No pericardial friction rubs were heard. The heartbeat was irregular, and the pulse rate was 75 beats per minute. Moist rales could be heard in both lower lungs. There was no edema or swelling in the lower extremities.
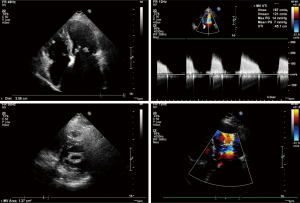
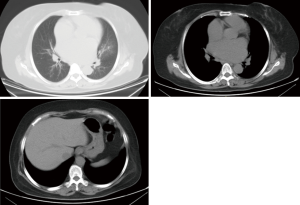
Transesophageal echocardiography at admission revealed moderate mitral stenosis with mild regurgitation; the left atrium, right atrium, and left ventricle were enlarged, along with a cloudy ultrasonic image of blood flow in the left atrium. Mild to moderate tricuspid regurgitation and slight aortic regurgitation were observed. The mitral valve was thickened, with increased echo enhancement. Furthermore, its opening was restricted, with a mitral valve area (MVA) of about 1.37 cm2. The peak transvalvular pressure was 14 mmHg, and the internal diameter of the annulus was 35.6 mm. Analysis of tricuspid regurgitation showed: pulmonary artery pressure, 36 mmHg; LVEF, 54%; LAd, 66 mm; LVIDd, 53 mm; AOd, 36 mm; and IVSd, 9.8 mm (Figure 1). ECG showed atrial fibrillation. Chest CT revealed left lung infection, enlarged left atrium, and the slightly thickened wall of gastric antrum (Figure 2). According to coronary angiography, the left main coronary artery (LM) showed no obvious stenosis, the left anterior descending artery (LAD) had interrupted plaque infiltration but had no obvious stenosis, and the circumflex coronary artery (LCx) and the right coronary artery (RCA) were generally normal. The carotid artery, blood vessels of the lower limbs, and the urinary system showed no obvious abnormality on ultrasound. Routine blood test and biochemical tests revealed C-reactive protein (CRP), 71.1 mg/L (<8.0); white blood cells (WBC), 3.9×109/L (3.5–9.5); red blood cells (RBC), 4.29×1012 g/L (3.8–5.1); HGB, 122 g/L (115–150 g/L); neutrophil ratio (NEUT%), 64.5% (40.0–75.0%); prothrombin time (PT), 17.4 s (11.0–14.5 s); activated partial thromboplastin time (APTT), 54.7 s (28–42 s); international normalized ratio (INR): 1.45 (0.8–1.2); creatine kinase, 76 U/L (≤167 U/L); troponin-I, 0.010 ng/mL (<0.100); total thyroxine (tT4), 93.0 ng/mL (70.0–152.5 ng/mL); thyroid-stimulating hormone, 2.64 (0.49–4.91) µIU/mL; free thyroxine, 0.8 ng/dL (0.6–1.3 ng/dL); and B-type natriuretic peptide (BNP), 326 (<120) pg/mL.
Treatments after her admission included oral anticoagulation therapy with warfarin, diuretic therapy including furosemide injection and spironolactone tablets, potassium supplementation, improvement of cardiac function, and anti-infection treatment. One week later, her chest tightness had improved, along with alleviated cough and production of yellow phlegm. She had no fever.
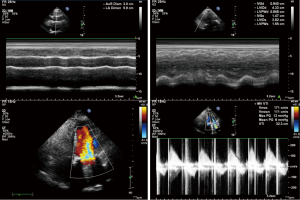
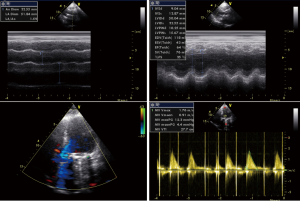
After admission, the patient had received anticoagulation, diuresis, and anti-infection treatments for 14 days. After her heart failure had improved and pulmonary infection disappeared, the patient had atrial fibrillation and left atrial enlargement, with left atrial thrombus visible during the operation. Under general anesthesia and CPB via femoral vessels, the patient underwent thoracoscopic-assisted mitral valve replacement via a small right thoracic incision + left atrial wall folding + left atrial thrombectomy. The surgery was unremarkable. The patient was observed in ICU for 24 hours and then transferred to the general ward. A second color Doppler echocardiography was performed one week after surgery (Figure 3). The patient was discharged on the 13th postoperative day and returned for a follow-up visit on the 23rd postoperative day (Figure 4).
iMDT discussion
The patient was admitted due to repeated chest tightness after exercise, shortness of breath, palpitations, and fatigue for more than 10 years, which had worsened over the past four days. The diagnosis at admission was rheumatic heart valve disease, which was manifested by moderate mitral stenosis with mild mitral insufficiency, atrial fibrillation, and pulmonary infection. She had undergone a CMC before. After anticoagulation, diuresis, and anti-infection treatments, her cardiac function and lung infection were improved. What is the next treatment plan?
Inputs from cardiovascular surgeons
Dr. Jian Xiao: The patient has a definite diagnosis of severe mitral stenosis, as manifested by clinical symptoms. She has indications for surgery. However, anti-inflammatory, cardiotonic, and diuretic therapies are still needed due to the presence of pulmonary infection and acute left heart failure. Surgical treatment is recommended only after the control of pulmonary infection and heart failure.
Balloon dilatation is not recommended since the patient had undergone CMC before and has a thickened mitral valve.
Either endoscopic-assisted or open surgery is feasible for this patient. However, since she has a poor cardiac function, an open procedure is safer for her. Thus, mitral valve replacement and tricuspid valvuloplasty are recommended.
Surgical precautions include (I) histidine-tryptophan-ketoglutarate (HTK) solution is recommended for myocardial protection; (II) because tricuspid valvuloplasty is required, the femoral vein and internal jugular vein, respectively, are recommended for venous cannulation; (III) and the adhesions should be carefully separated. After the surgery, (I) the heart rate shall be maintained around 90-100 beats/min; (II) the central venous pressure and pulmonary wedge pressure shall be monitored to control capacity and maintain appropriate preload, (III) and positive inotropic agents can be applied if necessary.
Dr. Peng Zhu: Surgical treatment is recommended based on the patient’s condition because the MVA is obviously reduced, along with obvious symptoms. Mitral valve replacement is recommended after the lung infection is controlled.
The patient had undergone CMC before, and her mitral annulus is densely and extensively calcified. Redo mitral valvuloplasty may lead to complications including a mitral valve tear, severe mitral insufficiency, and heart rupture. Therefore, mitral balloon valvuloplasty is not recommended. The surgical approach used in the previous CMC must have been the left pericardium and left atrial appendage via a left lateral incision; thus, there is no obvious mediastinal adhesion. Therefore, either open surgery or an endoscopic-assisted procedure is feasible for this patient.
Rheumatic mitral valve disease can be repaired in several centers in China. Since the general condition of this patient was not good, the duration of cardiopulmonary bypass should not be long; mitral valve replacement is recommended. Since the patient had undergone CMC before, pericardial adhesions are present, especially in the apex. Intraoperative mitral adhesions will not be found. Therefore, the intra- and post-operative management is the same as the conventional open-heart surgery for heart valve replacement.
Dr. Wenhui Gong: Active surgery is recommended for this patient. Anti-inflammatory, cardiotonic, and diuretic therapies should be offered before surgical treatment. After her cardiac function and pulmonary infection are improved, and there are no obvious symptoms of heart failure, surgical treatment can be considered. Warfarin should be discontinued for 5 days before operation, and low-molecular-weight heparin should be used for bridging until one day before surgery.
Balloon dilatation is not recommended for this patient because she had undergone CMC 30 years before, during which the valve must have been more flexible. At present, severe fusions have occurred at the valve junction and between subvalvular tendinous cords and papillary muscles. Thus, balloon dilatation is ineffective and highly risky for her. Color Doppler echocardiography has shown that some of the submitral structures have been fused. Therefore, open surgery is preferred as it has a shorter operating distance and thus is safer. Cardiopulmonary bypass (CPB) via femoral vessels can be considered. Thoracotomy is performed after CPB is established, so as to reduce the cardiac injury and harm caused by adhesion separation during the re-operation.
Mitral valve replacement is the recommended procedure, during which artificial mechanical mitral valve can be used. If a thoracoscopic-assisted surgery is performed, peripheral CPB should be established first. During the surgery, the mitral valve and the chordae tendineae that have been thickened and fused should be repaired at a long distance under the endoscope, so as to prevent myocardial injury during the suturing of the mitral valve and thus avoid ventricular rupture and other severe events. The postoperative management is generally the same as conventional surgery.
Dr. Chengxin Zhang: Surgical treatment is recommended for this patient since the patient has developed symptoms of heart failure, along with atrial fibrillation and structural changes of heart, which are indicated for surgery according to guidelines. The surgery should not be performed until the pulmonary infection, and heart failure are well controlled, and the patient is in a good general condition. Balloon dilatation is not recommended because the possibility of thrombosis cannot be ruled out. Selection of the surgical procedure depends highly on the experience and skill of a surgeon. For me, both the endoscopic-assisted operation for atrial fibrillation induction via a small incision on the right chest and the median approach can be applied. The femoral arteriovenous shunt is quite useful.
Based on the ultrasound findings, mitral valvuloplasty is preferred. Although the value of mitral valvuloplasty for the rheumatic mitral disease remains controversial, I prefer the application of mitral valvuloplasty, especially in younger patients who may have a higher quality of life and better left ventricular function. When the mitral valvuloplasty is performed, a medial approach can be used. Although there are adhesions, they can be separated via a femoral arteriovenous shunt, which is also fast and convenient. Since her left atrium is relatively large, the MAZE procedure is less effective. Therefore, internal suturing of left atrial appendage and tricuspid valvuloplasty will also be performed during the surgery. If an endoscopic-assisted surgery is performed, femorofemoral bypass is used. During cardiac fibrillation induction, the incision should be filled with carbon dioxide. The anatomical layers should be carefully identified to avoid accidental injury. Of course, the operation should not be performed if the operator has no knowledge or skill in endoscopic surgery or in making the median incision.
Inputs from cardiologists
Dr. Yue Liu: Treatments of rheumatic mitral stenosis include drug therapy, interventional therapy, and surgical treatment. Drug therapy is applied to prevent rheumatic fever, treat atrial fibrillation and heart failure, and reduce complications. Drug therapy provides palliation only. Active interventions should be adopted according to her medical condition, basic physical condition, and clinical manifestations. Moreover, she had moderate to severe mitral stenosis accompanied with obvious dyspnea and pulmonary hypertension, which require interventions including interventional and surgical procedures to relieve mitral stenosis and lower transvalvular pressure difference, so as to improve symptoms and quality of life and reduce mortality.
The indications for percutaneous balloon mitral valvuloplasty (PBMV) are expanding in European and American guidelines, and the procedure is the preferred surgical treatment of rheumatic mitral stenosis (4,5). However, this may be explained by the low incidence of rheumatic mitral stenosis in European and American countries, the main surgical intervention (closed dilatation), and the clinical endpoints of observation. Generally speaking, PBMV is more effective for patients (I) with an MVA of less than 1.5 cm2; (II) with soft valves and without moderate or severe mitral regurgitation; (III) with left atrial thrombosis and aortic valve disease; (IV) with ongoing dyspnea; (V) with rumble-like murmur and valvular sound in mitral valvular auscultation area during cardiac auscultation; and/or (VI) younger than 50 years.
The patient had progressive cardiac insufficiency (NYHA class III), which was aggravated by infection. Grade 2/6 apical diastolic rumble-like murmurs, which were conducted to the left axillary midline, was heard during cardiac auscultation. Color Doppler echocardiography has shown moderate mitral stenosis and soft valves, with an MVA of about 1.37 cm2. The peak transvalvular pressure was 14 mmHg. Analysis of tricuspid regurgitation showed: pulmonary artery pressure, 36 mmHg; LVEF, 54%; and other valves were slightly injured. Therefore, PBMV may have a better prognosis for the patient, with fewer amounts of trauma and no need for a second thoracotomy.
However, some authors have found that mitral stenosis patients accompanied by atrial fibrillation are less responsive to PBMV than those without atrial fibrillation (6). In our current case, the patient has enlarged left atrium, with cloudy echoes in the left atrium. Esophageal ultrasound is needed to clarify further whether there is thrombus in the left atrium. In addition, she had undergone CMC 30 years before, which resulted in valve adhesions and structural changes. All these factors affected the effectiveness of PBMV in this patient. Meanwhile, a new study confirms that PBMV increases the risk of severe mitral regurgitation in these patients (7). After carefully reviewing the medical records of this patient, we find the NYHA cardiac function, MVA, anterior and posterior diameters of the left atrium, and atrial fibrillation meet the indications of surgical treatment. Based on the specific conditions in China, surgical treatment is a good option for patients with mitral stenosis. Inputs from both surgeons and physicians are needed. STS and Euroscore scoring, surgical risk assessment, and prediction of long-term prognosis after surgery are needed. Furthermore, since the patient had undergone CMC and thus may have mediastinal adhesions and anatomical abnormalities. Open surgery is recommended, during which left atrial appendage closure is recommended to reduce the incidence of thromboembolism after the operation. In my opinion, surgical treatment is preferred for this patient since it may completely heal the stenosis, resolve the co-existing atrial fibrillation, and have good cost-effectiveness.
Inputs from intensivists
Dr. Yuetian Yu: The patient is a middle-aged woman with preoperative cardiac insufficiency complicated with pulmonary infection. She needs to be transferred to the intensive care unit (ICU) for the monitoring of hemodynamics, respiratory function, urine volume, and drainage flow after cardiac valve surgery.
Monitoring of circulatory system: Since cardiopulmonary bypass can affect some factors of the body, including: (I) myocardial injury, which may affect the myocardial contractility; (II) intraoperative blood loss, which can cause changes in the blood volume of the whole body; (III) change in peripheral vascular resistance, caused by low temperature and other factors, which will also cause changes in blood pressure. Therefore, hemodynamic measurements should include heart rate, arterial pressure, central venous pressure, pulmonary floating catheter-related indicators, and bedside echocardiography. The patient has a history of rheumatic heart disease, and her preoperative cardiac function has been impaired to some extent. It is suggested that the heart rate should be maintained at 100 beats/min after an operation to maintain cardiac output. Any arrhythmia should be carefully monitored.
Monitoring of respiratory system: The patient has cough and cough up yellow and purulent sputum before surgery. Since there is a lesion in the left lower lobe, bacterial pneumonia cannot be excluded. Sputum smear and culture should be used before surgery to identify the pathogens causing pulmonary infection. After the cardiac surgery, the patient’s ability to coughing up the sputum will be restricted due to pain and bedriddenness, and the lung infection may recur or worsen. The third-generation cephalosporins (e.g., ceftazidime) or quinolones (e.g., levofloxacin) may be used as anti-infective regimens after the cardiac surgery. Patients should be encouraged to become ambulatory early, so as to promote sputum drainage.
After cardiac surgery, high-flow nasal cannula (HFNC) can be used to ensure oxygen supply and reduce the risk of re-intubation due to an insufficient oxygen supply. Therefore, it is suggested that HFNC can be applied 3 days after the operation. If there is no obvious hypoxia symptom after 3 days, the nasal catheter can be used for oxygen inhalation.
Monitoring of urine volume and drainage: Observation of urine volume is important after cardiac surgery because urine volume usually reflects the cardiac function, effective circulating volume, and renal function. Typically, the urine volume after cardiac surgery should be larger than 0.5–1 mL/h/kg. The reasons for a smaller urine volume should be analyzed based on other monitoring indicators. In addition, the drainage volume should also be observed; if the hourly drainage flow is greater than 200 mL, the possibility of active bleeding should be considered. The possibility of abnormal blood coagulation function or the need for further surgical treatment will be further clarified.
Inputs from anesthesiologists
Dr. Chunguang Wang: The patient had undergone CMC 30 years before. Mitral stenosis recurred recently, with an MVA of about 1.37 cm2. The patient also suffers from decompensated heart failure. Therefore, the patient currently has surgical indications and should receive surgical treatment.
The patient had cardiac insufficiency accompanied by pulmonary infection. Thus, cardiac and pulmonary functions must be actively adjusted before the operation. Pulmonary infection should be fully controlled for three months before the operation. Balloon dilatation of the mitral valve is expected to be ineffective in this patient. Mitral valve replacement is recommended.
An endoscopic-assisted procedure is preferred. The reasons are as follows: (I) an endoscopic-assisted surgery is less invasive and will have fewer impacts on the pathological and physiological functions; (II) the patient has developed cardiac insufficiency, and an open surgery carries a high risk for her, and (III) the patient currently has plaque infiltration in middle LAD. If the disease progresses rapidly in its later stage, the patient will have to receive a bypass surgery for multiple coronary artery lesions. Endoscopic surgery has less damage to the heart and its surrounding structures than open surgery, which makes the bypass surgery possible.
The surgical precautions include (I) cardiopulmonary bypass via femoral vessels is established. After the ascending aorta is blocked under endoscopy, mitral valve replacement is performed. (II) A lateral incision is made. Since the patient has a relatively large left atrium, the atrial sulcus approach can be used. (III) The intra- and post-operative anesthesia is similar to those of an open surgery; the only difference is that an endoscopic operation has small surgical trauma, and fast-track cardiac anesthesia can be applied to accelerate the early recovery of the patient.
Question 1: Which treatment would you choose, open or minimally invasive surgery?
Expert opinion 1: Irbaz Hameed and Arash Salemi
In most patients experiencing restenosis after surgical commissurotomy, mitral valve replacement is recommended. However, percutaneous mitral commissurotomy can be proposed in select patients if the predominant mechanism is commissural refusion (8). Our young patient underwent CMC and experienced symptomatic mitral stenosis recently. The patient also showed signs of decompensated heart failure in the setting of pulmonary infection. Consequently, surgical mitral valve replacement is recommended in this patient following resolution of pulmonary infection and cardiovascular optimization.
The choice of open vs. minimally invasive mitral valve replacement is patient specific and dependent on surgeon experience. Minimally invasive mitral valve replacement has comparable short and long-term outcomes to conventional mitral valve surgery (9). While it has some disadvantages of prolonged cross clamp time, CPB time, and procedure time, its advantages include reduced sternal complications, blood transfusions, duration of ventilation, and intensive care unit and hospital length (10). In the present patient, there is no obvious contraindication to minimally invasive mitral valve replacement considering the previous CMC may have likely been via a left lateral incision with mediastinal adhesions unlikely. Moreover, minimally invasive mitral valve replacement is associated with a lower rate of atrial fibrillation and valve-related reoperations (11). In the hands of an experienced high-volume surgeon, it may be a suitable approach for our patient who is receiving anti-coagulation therapy, in heart failure, and susceptible to atrial fibrillation. Moreover, the plaque infiltration of the LAD seen on coronary angiography can evolve into significant disease in the future which may require sternotomy for coronary artery bypass grafting surgery. Open replacement can also be performed with excellent outcomes if the surgeon is more comfortable with that.
Expert opinion 2: Daniel Hernandez-Vaquero
This is a patient with moderate rheumatic mitral stenosis who had already undergone commissurotomy 30 years ago. This valvulopathy is probably the cause of the patient's symptoms for 10 years. Mitral valve area <1.5 cm2 and symptoms indicate surgery. If possible, this surgery must be performed once the infection and pulmonary edema are solved. In this sense, medical performance was completely adequate. The patient is young so surgery must consist at least of mitral valve replacement by using a mechanical prosthesis.
If tricuspid annuloplasty is needed, open surgery is the preferred approach. If not, minimally invasive mitral valve replacement using a mechanical prosthesis and preserving the posterior mitral apparatus would be, under my point of view, the most indicated operation. The preservation of the anterior apparatus is problematic since chordae tendineae will be thickened and retracted and may block the prosthesis valves.
Expert opinion 3: Taufiek Konrad Rajab
In this case, the optimal approach is dictated by the previous operation to relieve stenosis of the mitral valve and the presence of left atrial thrombus. The 2014 guidelines of the American College of Cardiology for symptomatic rheumatic mitral stenosis with MVA <1.5 cm2 in the absence of left atrial thrombus or moderate mitral regurgitation and favorable valve morphology would strongly suggest percutaneous mitral balloon commissurotomy. However, the prior mitral commissurotomy means that the valve morphology is likely unfavorable for mitral balloon commissurotomy. Moreover, echocardiography showed a cloudy left atrial image and the authors had to perform a left atrial thrombectomy. Therefore mitral balloon commissurotomy is contraindicated and the patient should undergo mitral valve replacement (12).
In order to replace the mitral valve, a minimally invasive thoracotomy is an excellent approach to avoid mediastinal adhesions from the previous operation, but only if there are no right pleural adhesions. In my opinion, the thoracoscopic assisted approach chosen by the authors is an ingenious solution for this problem. By starting the operation with an exploratory right thoracoscopy, the density of pleural adhesions can be evaluated before committing to a minimally invasive thoracotomy. If exploratory right thoracoscopy reveals dense pleural adhesions then I would convert to an open sternotomy approach.
Expert opinion 4: Francesco Nappi
Morphology and function of the mitral valve with previous CMC
Rheumatic mitral disease due to bacterial streptococcal infection can cause restricted leaflet motion from thickening of the leaflet edges and subvalvular apparatus with a doubling in increasing cause of mild to moderate mitral regurgitation among patients with previous CMC procedure for rheumatic heart disease and mitral stenosis (13-15). As described by Carpentier in this case we find a type IIIa MR with leaflet restriction in diastole, most commonly associated to mitral valve stenosis (16). In these patients it is important to be assess the presence, degree, and localization of calcium in the annulus, which can create complications during the standard mitral valve surgery procedure and even more when the choice of minimally invasive operation is preferred (17).
In this case report the recipient of thoracoscopic-assisted mitral valve replacement has a rheumatic MV disease (Carpentier type IIIa) that is carefully diagnosed by noting leaflet thickening, especially at the leaflet edges and commissures, usually with some degree of narrowing of the mitral valve (14,16,17). We can observe thickening and shortening of the chordal apparatus, resulting in retraction of the leaflets in both systole and diastole (14). Transthoracic Echocardiography (TTE) based assessment shows that the anterior leaflet assumes “hockey stick morphology” while the posterior leaflet is fixed (16).
Practice guidelines recommend consideration of mitral-valve surgery for patients with severe primary mitral regurgitation with LVEF less than or equal to 30% and who are symptomatic (Stage D) despite the best available medical therapy. As we note in the clinical case, although the patient does not have severe mitral regurgitation, he can nevertheless be classified as Class IIb level C because moderate stenosis is coupled with moderate mitral valve insufficiency. In case of rheumatic etiology (Carpentier type IIIa) mitral valve repair should be performed by highly experienced surgeons who have dedicated their surgical career to MV repair (5).
In our thirty years of experience we have used a partial mitral homograft to repair the anterior leaflet. Alternatively, we have chosen an autologous pericardial patch to reconstruct mitral morphology and ensure normal leaflet coaptation (14,15,18,19) (Figure 5).
The guidelines and professional society recommendations do not specify whether to perform standard mitral valve surgery or minimal invasive procedure, because conclusive evidence is lacking to indicate which of these interventions is superior (5).
First, we prefer to use a standard approach for patients who receive a reoperation for the progression of rheumatic mitral valve disease due to streptococcal endocarditis (15). We might speculate that decisions regarding the optimal treatment of reoperation of mitral valve after previous CMC are based on multiple variables, including complexity of mitral valve disease and severity, hemodynamic consequences, disease stage, patient comorbidities, and the experience of the heart valve team and its members (14,15).
Second, we believe that evidence-based management can be helped by the use of an algorithm (16) We strongly suggest the use of a precise standardized nomenclature when reporting echocardiographic results, which is very useful for guiding surgical/interventional decision making (15,16). For example in primary MR with Carpentier type IIIA leaflet motion associated with mitral stenosis, the surgical spectrum includes focal or diffuse leaflet and subvalvular thickening and commissural fusion due to rheumatic heart disease. The papillary muscles are calcified and tenaciously adherent to the ventricular muscle. The chordae tendinae are fused particularly at the level of the anterior and posterior commissures, often making mobilization and resection of leaflets more difficult. In most cases the posterior leaflet cannot be spared and a total mitral valve resection is recommended to ensure the best size of the prosthesis to be implanted (14,17).
Third, during the operation it is possible to exert a strong traction on the papillary muscles with consequent tearing of the ventricular muscle. Furthermore, the removal of calcifications of the mitral annulus can cause serious damage to the trigonal ventricular muscle and to the commissures of the mitral valve. The result of these serious impairment can be the rupture of the heart (15).
Finally, concerning the patient described in the case report we choose a standard approach for mitral valve surgery because we prefer to replace the anterior mitral valve leaflet with the use of a partial homograft (14,15). Alternatively, we reconstruct the mitral valve with the use of an autologous pericardium patch, which serves to enlarge the anterior leaflet by solving the morphological condition of Carpentier type IIIA leaflet motion (abnormal leaflet motion by definition) (19) (Figure 5).
Question 2: In this patient, will you perform tricuspid valvuloplasty during surgery? Why?
Expert opinion 1: Irbaz Hameed and Arash Salemi
Secondary tricuspid regurgitation is an independent factor associated with decreased survival (20). Mitral valve replacement decreases pulmonary vascular pressure and reduces right ventricular overload and conservative management of moderate secondary tricuspid regurgitation has been suggested (21). However, left-sided valve disease and secondary tricuspid regurgitation are not linearly related patho-physiologically (22). The presence of untreated moderate-to-severe tricuspid regurgitation at the time of mitral valve surgery is associated with reduced postoperative survival (23) and worse quality of life (24). Importantly, redo surgery for tricuspid regurgitation is associated with a high operative mortality of 10–25% (25). Therefore, in cases such as our patient with moderate-to-severe tricuspid regurgitation in the setting of mitral valve disease are generally recommended to undergo tricuspid valve surgery/valvuloplasty at the time they undergo mitral valve surgery.
Expert opinion 2: Daniel Hernandez-Vaquero
The need for tricuspid annuloplasty is unclear.
Tricuspid annuloplasty as a concomitant procedure of mitral valve surgery is indicated in case of: severe tricuspid regurgitation (26) or mild to moderate tricuspid regurgitation and annulus dilatation (27) or the presence of signs or symptoms of right ventricular function.
In this case, because the symptoms of left heart failure appeared 10 years ago, it is likely that some degree of right ventricular dysfunction or dilatation exists. Despite no signs or symptoms of right ventricular failure are reported, prothrombin time was abnormal which can be indicative of liver failure and this may be of cardiological cause. If this is the case, tricuspid annuloplasty is needed. Even in the case that this abnormality is due to oral anticoagulants and no other sign or symptoms of right ventricular failure exist, tricuspid annuloplasty is indicated if the annulus is >40 mm or >21 mm/m2.
So, the only rare case where a tricuspid annuloplasty is not indicated as a concomitant procedure of a mitral valve replacement would be a patient without severe tricuspid regurgitation and no signs or symptoms of right ventricular function, no right ventricular dilation or dysfunction and without annulus dilatation.
Expert opinion 3: Taufiek Konrad Rajab
In this patient, tricuspid valvuloplasty is indicated even though tricuspid regurgitation is only mild to moderate because of the concomitant left sided valve surgery and because there is pulmonary hypertension according to the guidelines of the American Heart Association and American College of Cardiology (12). The ejection fraction of 54% is only mildly depressed and therefore the patient is likely to tolerate the additional cross-clamp time well. If there is concern for myocardial protection and cross-clamp time, then tricuspid valve repair could be attempted on the beating heart.
Expert opinion 4: Francesco Nappi
As described by the authors in transesophageal echocardiography. The patient has mild to moderate tricuspid regurgitation with enlarged right atrium. In these patients the failure of the tricuspid valve is never completely functional or secondary regurgitation. In fact, the valve can present a different degree of structural degeneration of the free edge of the leaflet consequent to the primary localization of the streptococcal infection. In our experience we prefer to perform a tricuspid valve repair to avoid running into a “forgotten tricuspid” status that often require a newly reoperation (13,15,16). Standard surgery allows, through Guy Redorn’s biatrial technique, to easily access the mitral valve and the tricuspid valve. The operation can be carried out in a reasonable time without exposing the patient to additional risks connected to longer interventional times due to the minimally invasive technique chosen by the surgeon to treat more than one valve (15).
Question 3: What should be noted during minimally invasive mitral valve replacement in this patient?
Expert opinion 1: Irbaz Hameed and Arash Salemi
Care must be taken to minimize risk of site-related complications. If the procedure is performed by partial or hemi-sternotomy, there is a risk of injury to the right internal mammary artery and vein. These must be carefully identified to prevent post-operative bleeding and re-exploration. Thoracotomy and parasternal incisions may be associated with wound complications, including incisional and lung hernias, phrenic injury, dehiscence, and infection (28). There is also a risk of lung re-expansion reperfusion injuries with single ventilation performed with cardiopulmonary bypass (29).
In our patient, there is a possibility of conversion from minimally invasive incision to full sternotomy. These can occur pre-cardiopulmonary bypass due to poor visualization and possible adhesions, and the patient and her family should be informed of this potential risk along with other risks during informed consent.
The pericardium and multiple port access incisions must be carefully inspected and drained during the procedure to avert impaired drainage which can cause postoperative subacute tamponade or pericardial effusion in the presence of excess postoperative mediastinal drainage. Also, a complete evacuation of trapped air in the left ventricle must be ensured by transesophageal echocardiography monitoring and carbon dioxide (CO2) field insufflation (denser and more soluble than air).
Expert opinion 2: Daniel Hernandez-Vaquero
An active search for thrombus in the left atrial appendage should be performed. Ligation of this appendage has shown no clear benefit to date. However, the results of the clinical trial Left Atrial Appendage Occlusion Study III (LAOOS III) will be published soon and will shed light on this issue.
Expert opinion 3: Taufiek Konrad Rajab
Due to the previous mitral valve commissurotomy and rheumatic disease, there should be a high index of suspicion for mitral annular calcification. Luckily, the computed tomography in Figure 2 does not show evidence of severe calcification. I agree with Dr. Liu and Dr. Zhang that the left atrial appendage should be addressed to minimize the risk of embolic complications from atrial fibrillation. This is particularly important because the authors encountered left atrial thrombus. If a median sternotomy approach is chosen and there are minimal mediastinal adhesions then the atrial appendage can be excised. Otherwise it should be closed from inside the left atrium.
Expert opinion 4: Francesco Nappi
The severity of the lesions and the pathoanatomic approach can lead to the success of minimally invasive mitral valve surgery in rheumatic disease (14,17). In these patients a mitral valve with limited impairment will result in successful replacement that is achievable by the majority of cardiac surgeons. Mitral valve surgery using minimal invasive procedure becomes more difficult if there is massive calcification of the annulus, or calcification and fibrosis of the valve leaflets with involvement of fused commissure and chordae tendinae precluding adequate removal of entire mitral valve apparatus (14,15,17).
The primary objective of this surgery is to ensure the same results as the standard approach, namely guarantee an optimal valve size that avoids a high post-operative transvalvular gradient.
The secondary objective is to not leave a residual insufficiency of the tricuspid requiring a reoperation (5,15,16).
Finally, the choice of prosthesis valve it is not free from some considerations. I did not find which valve between conventional mechanical or stented prosthesis was used by the authors. For noninfectious indications, the Duke group favors mechanical over bioprosthetic valves when replacement of the mitral valve is recommended. Conventional stented xenograft in used in only 8% of mitral valve procedures compared with 33% mechanical and 59% repairs (30).
The presence of atrial fibrillation favors the use of the mechanical valve because the patient already has an anticoagulant treatment but we do not exclude the use of biological valves if preferred and indicated by the lifestyle. Today, for these patients the future use of the trans mitral valve therapy is a topic of discussion in the heart team shared decision making (31).
Conclusions
Multi-disciplinary care can enhance the ability of hospitals to diagnose and treat difficult diseases, ensure healthcare safety, and, to a certain extent, reduce medical risks. Compared with the first cardiac valve surgery, the redo operation has higher risks (32), which can be explained by the poorer preoperative cardiac performance, increased surgical difficulty, and a higher risk of infection. Based on the inputs of experts from the departments of cardiovascular surgery, cardiology, critical care medicine, and anesthesiology, our current patient finally underwent thoracoscopic-assisted mitral valve replacement via small right thoracic incision + left atrial wall folding + left atrial thrombectomy. The postoperative recovery was good. Multi-disciplinary care can help doctors to make correct and comprehensive treatment decisions that are more conducive to the rehabilitation of patients. For high-risk surgical patients, the use of multidisciplinary care is highly valuable.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- McKay RG, Lock JE, Safian RD, et al. Balloon dilation of mitral stenosis in adult patients: postmortem and percutaneous mitral valvuloplasty studies. J Am Coll Cardiol 1987;9:723-31. [Crossref] [PubMed]
- Malvindi PG, van Putte BP, Leone A, et al. Aortic reoperation after freestanding homograft and pulmonary autograft root replacement. Ann Thorac Surg 2011;91:1135-40. [Crossref] [PubMed]
- Fukunaga N, Okada Y, Konishi Y, et al. Impact of tricuspid regurgitation after redo valvular surgery on survival in patients with previous mitral valve replacement. J Thorac Cardiovasc Surg 2014;148:1983-8. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg 2014;148:e1-132. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70:252-89. [Crossref] [PubMed]
- Tarka EA, Blitz LR, Herrmann HC. Hemodynamic effects and long-term outcome of percutaneous balloon valvuloplasty in patients with mitral stenosis and atrial fibrillation. Clin Cardiol 2000;23:673-7. [Crossref] [PubMed]
- Tyczyński P, Chmielak Z, Ruzyllo W, et al. Percutaneous mitral balloon valvuloplasty: beyond classic indications. Kardiol Pol 2018;76:845-51. [Crossref] [PubMed]
- Song H, Kang DH, Kim JH, et al. Percutaneous mitral valvuloplasty versus surgical treatment in mitral stenosis with severe tricuspid regurgitation. Circulation 2007;116:I246-50. [Crossref] [PubMed]
- Falk V, Cheng DC, Martin J, et al. Minimally invasive versus open mitral valve surgery: a consensus statement of the international society of minimally invasive coronary surgery (ISMICS) 2010. Innovations (Phila) 2011;6:66-76. [Crossref] [PubMed]
- Ito T. Minimally invasive mitral valve surgery through right mini-thoracotomy: recommendations for good exposure, stable cardiopulmonary bypass, and secure myocardial protection. Gen Thorac Cardiovasc Surg 2015;63:371-8. [Crossref] [PubMed]
- Cheng DC, Martin J, Lal A, et al. Minimally invasive versus conventional open mitral valve surgery: a meta-analysis and systematic review. Innovations (Phila) 2011;6:84-103. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:2440-92. [Crossref] [PubMed]
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [Crossref] [PubMed]
- Olivito S, Lalande S, Nappi F, et al. Structural deterioration of the cryopreserved mitral homograft valve. J Thorac Cardiovasc Surg 2012;144:313-20, 320.e1.
- Nappi F, Spadaccio C, Dreyfus J, et al. Mitral endocarditis: a new management framework. J Thorac Cardiovasc Surg 2018;156:1486-1495.e4. [Crossref] [PubMed]
- Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303. [Crossref] [PubMed]
- Silbiger JJ. Anatomy, mechanics, and pathophysiology of the mitral annulus. Am Heart J 2012;164:163-76. [Crossref] [PubMed]
- Nappi F, Spadaccio C, Acar C. Use of allogeneic tissue to treat infective valvular disease: Has everything been said? J Thorac Cardiovasc Surg 2017;153:824-8. [Crossref] [PubMed]
- Aubert S, Flecher E, Rubin S, et al. Anterior Mitral Leaflet Augmentation With Autologous Pericardium. Ann Thorac Surg 2007;83:1560-1. [Crossref] [PubMed]
- Schueler R, Ozturk C, Sinning JM, et al. Impact of baseline tricuspid regurgitation on long-term clinical outcomes and survival after interventional edge-to-edge repair for mitral regurgitation. Clin Res Cardiol 2017;106:350-8. [Crossref] [PubMed]
- Braunwald NS, Ross J Jr, Morrow AG. Conservative management of tricuspid regurgitation in patients undergoing mitral valve replacement. Circulation 1967;35:I63-9. [Crossref] [PubMed]
- Unger P, Clavel MA, Lindman BR, et al. Pathophysiology and management of multivalvular disease. Nat Rev Cardiol 2016;13:429-40. [Crossref] [PubMed]
- Taramasso M, Vanermen H, Maisano F, et al. The growing clinical importance of secondary tricuspid regurgitation. J Am Coll Cardiol 2012;59:703-10. [Crossref] [PubMed]
- Groves PH, Lewis NP, Ikram S, et al. Reduced exercise capacity in patients with tricuspid regurgitation after successful mitral valve replacement for rheumatic mitral valve disease. Br Heart J 1991;66:295-301. [Crossref] [PubMed]
- King RM, Schaff HV, Danielson GK, et al. Surgery for tricuspid regurgitation late after mitral valve replacement. Circulation 1984;70:I193-7. [PubMed]
- Alfieri O, De Bonis M. Tricuspid valve surgery for severe tricuspid regurgitation. Heart 2013;99:149-50. [Crossref] [PubMed]
- Dreyfus GD, Corbi PJ, Chan KM, et al. Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann Thorac Surg 2005;79:127-32. [Crossref] [PubMed]
- Ng PC, Chua AN, Swanson MS, et al. Anterior thoracotomy wound complications in minimally invasive direct coronary artery bypass. Ann Thorac Surg 2000;69:1338-40; discussion 1340-1. [Crossref] [PubMed]
- Sugiyama Y, Shimizu F, Shimizu S, et al. Severe Re-expansion Pulmonary Edema Induced by One-Lung Ventilation. Respir Care 2015;60:e134-40. [Crossref] [PubMed]
- Daneshmand MA, Milano CA, Rankin JS, et al. Influence of Patient Age on Procedural Selection in Mitral Valve Surgery. Ann Thorac Surg 2010;90:1479-85; discussion 1485-6. [Crossref] [PubMed]
- Nappi F, Attias D, Avtaar Singh SS, et al. Finite element analysis applied to the transcatheter mitral valve therapy: Studying the present, imagining the future. J Thorac Cardiovasc Surg 2019;157:e149-51. [Crossref] [PubMed]
- Jamieson EW, Geldrop MWV, Ye J, et al. 479 Patient outcome after MVR with mechanical or bioprostheses: Do the benefits of a bioprosthesis outweigh the reoperation risk? Can J Cardiol 2011;27:S236. [Crossref]


