
Health-related quality of life and survival among 10-year survivors of esophageal cancer surgery: gastric tube reconstruction versus whole stomach reconstruction
Introduction
Esophageal cancer is the eighth most common type of cancer and the sixth most common cause of cancer-related mortality (1). Tumor recurrences typically occur within the first year after surgery, and are usually followed by rapid death (2). Survival at 1 year, 3 years and 5 years after surgery was 46.5%, 24.1%, and 19.7% respectively in 1987–1991. In 1997–2000, the survival at 1, 3 and 5 years improved to 61.7%, 39.9%, and 30.7%, respectively (3). Despite improved survival during the past decades, the 5-year survival is still relatively low in patients undergoing curatively intended surgery. The 10-year survival is barely reported and little is known about the factors influencing the long-term (10-year) survival. Although the long-term survival remains low, esophagectomy is still the key curatively intended treatment for patients with esophageal cancer. Esophagectomy is a highly invasive procedure with a 50% risk of severe postoperative complication within 30 days of surgery (4). For this disease with comparatively poor prognosis and high incidence of postoperative complications, survival alone cannot be able to adequately describe outcome. Nowadays, health-related quality of life (HRQL) has been paid more and more attention. Esophagectomy greatly impacts on emotional, physical and social health. It has highly negative and long-lasting consequences for the patient’s HRQL. Some studies have reported a recovery of HRQL to preoperative levels at 3 years after surgery (5); others reported that certain patients could even have substantially worse HRQL within 5 years of surgery (6). Factors influencing HRQL after surgery is still in debate. Our previous research indicated that patients with gastric tube reconstruction may present a better HRQL within 5 years of surgery, but it is unclear whether this recovery persists over time.
Reconstruction after esophagectomy is one of the most important procedures during surgery for esophageal cancer. Two major methods for digestive tract reconstruction are applied: narrow gastric tube (NGT) reconstruction and whole stomach (WS) reconstruction. In 1972, Akiyama proposed the concept of NGT reconstruction firstly (7). It is applied worldwide now (8). Although there have been several reports comparing gastric tube with WS on blood flow and short-term HRQL (9,10), little is known about its impact on long-term (10 years) HRQL and survival.
Methods
Study design
Participants in this study included 104 of 112 patients, 81 men and 23 women (median age: 60.09 years) for esophageal cancer between 2007 and 2008. The patients were evaluated by esophagography, esophagoscopy, bronchoscopy, computed tomography (CT) and positron emission tomography (PET). Patients with tumours infiltrating into adjacent organs (bronchus, aorta, etc.) or with distant metastases (brain, bone, liver, etc.) were excluded from this study. A total of 104 patients were include finally and they were randomized by envelop method to receive either NGT reconstruction (NGT group, n=52) or WS reconstruction (WS group, n=52) (11). All patients signed the consent, and the ethics committee approved this study. Site of anastomosis was decided by the location of tumor: cervical manual anastomosis for tumor located in the upper one-third of the esophagus and stapled intrathoracic anastomosis for tumor located in the lower two-thirds of the esophagus. The NGT was formed from the distal aspect of the lesser curvature of the stomach with application of linear staplers by resection of the lesser curvature of the stomach. The formation of the gastric conduit (about 3 cm in diameter) was based on the preservation of the gastroepiploic vessels of the greater curvature of the stomach. The WS reconstruction was performed after the stomach was adequately mobilized, and an anastomosis between the end of the esophagus and the fundus of the stomach was created. Irrespective of the site of anastomosis, all gastric tubes were placed in the posterior mediastinum. The postoperative chemotherapy of docetaxel and cisplatin was provided to all patients.
Clinical data collection
The questionnaire includes 25 items of HRQL, with reference to EORTC-QLQ-C30 (version 3.0) and QLQ-QES18. It was delivered to the patients at 3 weeks, 6 months, 1 year, 2 years, 5 years and 10 years after surgery. The QLQ-C30 contains scales and items addressing functional aspects of HRQL, and symptoms that commonly occur in patients with cancer. The QLQ-QES18 was designed especially for patients with esophageal cancer undergoing surgery. In the symptom scales and single items, a high score means more severe symptoms. In the functional scales, a high score means function. For example, for the ‘dysphagia’, the question is ‘have you had trouble with swallowing food, or choked when swallowing?’. If the answer is ‘not at all’, it is scored 1; if the answer is ‘a little’, it is scored 2; if the answer is ‘quite a bit’, it is scored 3; if the answer is ‘very much’, it is scored 4. All scale and item scores are linearly transformed into a 0–100 scale. Score of the ‘global HRQL’ >30 was considered harboring a good HRQL. Patients completed this questionnaire at 3 weeks, 6 months, 1 year, 2 years, 5 years and 10 years after surgery. The survival data were also collected at each follow-up, including survival status (alive/death), reason of death, surviving time, etc.
Statistical analyses
The SPSS 18.0 was used for statistical analyses. Unpaired t tests were used for the differences between groups. Nominal data were calculated with the chi-square test. P<0.05 was considered as statistically significant.
Results
Patient characteristics
Of 104 patients, 103 were followed up until death, or the end of the study. Their average age was 60.1±6.8 years. The follow-up rate was 99%. No significant differences in age, sex, FEV1 (%), preoperative diet, tumor site, histopathology and TNM staging was found between groups (Table 1).
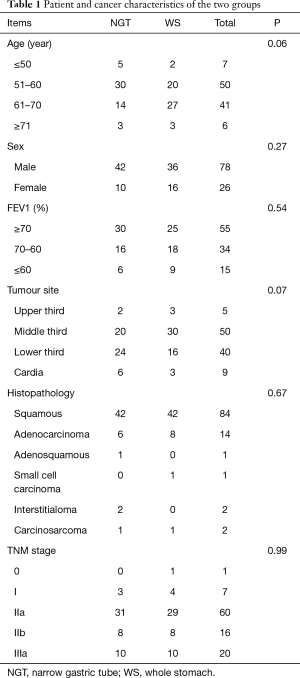
Full table
Surgical data and postoperative complications
All patients were operated (52 in NGT group and 52 in WS group). Stapled intrathoracic anastomosis was performed on 91 patients. Cervical manual anastomosis was performed on 13 patients. Regarding to the site of anastomosis, operation time (h), blood loss (mL), ICU stay (d), gastrointestinal decompression (mL) and chest drainage (mL), no significant difference between the two groups was identified (Table 2). Major postoperative complications were summarized as follow: for the postoperative reflux esophagitis (RE), there are three patients in NGT group and 11 patients in WS group (P=0.04); for the anastomotic hemorrhage, there was one patient in NGT group; for the damage of the recurrent laryngeal nerve, there was one patient in NGT group; one case of chylothorax and two cases of pulmonary function impairment were found in WS group (Table 2). The complication rate was 26.9% in NGT group and 48.1% in WS group (P>0.05). All hospital mortality (30 days after surgery) occurred in WS group: one patient died of pneumonia, and another died of stress ulcer bleeding.
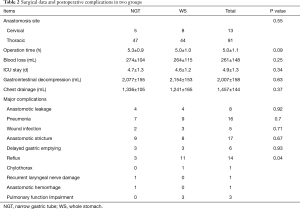
Full table
Overall survival
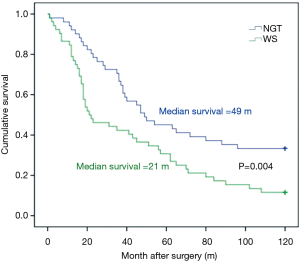
Thirteen patients (4 in NGT group and 9 in WS group) died of cachexia, multiple-organ failure and other nutrition-related complications during the 1-year follow-up. The overall 1-year survival was 88% (92% for NGT group and 84% for WS group, P=0.36). During the 2 years follow-up, 44 of the 104 patients died (15 in NGT group and 29 in WS group). Most deaths were caused by tumor recurrence and metastasis, except one dead of traffic accident. The overall 2-year survival was 50% (56% for NGT group and 44% for WS group, P=0.14) (12). During the 5 years follow-up, 70 of the 104 patients died (33 in NGT group and 37 in WS group). The overall survival was 36% (42% for NGT group and 27% for WS group, P=0.027) (13). During the 10 years follow-up, 81 of the 104 patients died (35 in NGT group and 46 in WS group). The 10-year overall survival was 22.3%. The 10-year survival of patients in NGT group was 33.3% and in WS group 11.5% (P=0.004) (Figure 1). The recurrence and metastasis rate in NGT group and WS group at 10 years was 67.3% and 88.5%, respectively.
Items and scales on HRQL: changes over time
Questionnaires were sent to patients at 3 weeks, 6 months, 1 year, 2 years, 5 years, and 10 years after surgery. Reflux was significantly less in NGT group at 3 weeks, 6 months and 1 year. However, this advantage fades out during 5–10 years. Nausea is the only notable symptom that was significantly worse in WS group at 2 years after surgery, and it also resolved during the follow-up of 5 to 10 years. The difference of dysphagia was significant at 6 months and vanished during 2 years. However, it reoccurred at 5 years and remains the only symptom that was significantly worse in WS group at 10 years follow-up (Table 3).
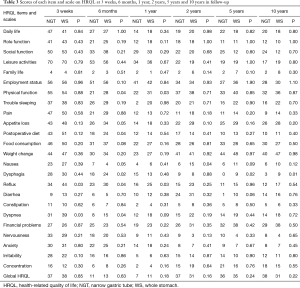
Full table
Number of patients presenting good HRQL: changes over time
Score of the item ‘global HRQL’ >30 was considered harboring a good HRQL. There are significantly more patients presenting good HRQL in NGT group in early-term follow-up (6 months and 1 year after surgery) (P<0.05). During the following 2–10 years, this number between groups did not show significant differences (Table 4).

Full table
Discussion
Results from this study showed that in contrast with WS reconstruction, patients with gastric tube reconstruction suffered less digestive tract complications, had a quicker recovery and a better HRQL in early-term follow-up (6 months and 1 year after surgery). This recovery resolved in both groups during the follow-up of 2 years and since then, no significant difference on number of patients presenting good HRQL was found. HRQL recovers to a level comparable between groups in most patients who survive 10 years after esophagectomy for cancer (Tables 3,4). The difference on HRQL in early-term follow-up may be explained by the occurrence of major postoperative complications. In our study, patients in NGT group presented significantly less reflux than patients in WS group (Table 2). This is consistent with the result of a population-based prospective study about 5-year survivors of esophageal cancer surgery (2), in which the occurrence of major postoperative complications was proved to be an independent predictor for poor HRQL. Symptoms like dyspnea, fatigue, and eating restrictions were clinically and statistically significantly deteriorated throughout the follow-up in patients with major postoperative complications compared with patients without major complications. Inflammatory molecules were used as an explanation for the mechanism (2). Furthermore, our study proved that major postoperative complications may exert a negative effect on HRQL but it is ‘temporary’: when the follow-up is ‘long’ enough (10 years), the differences on HRQL will finally resolve.
Another interesting phenomenon found during 10 years follow-up is that all of the six patients alive in WS group complain the problem of dysphagia, while few patients in NGT group complain of that (Figure 2). This is consistent with the result of our investigation at 5 years after surgery (13). One possible explanation is that dysphagia is always related to malignant process of locoregional or endoluminal tumor recurrence. Patients under NGT reconstruction have a significantly better survival at 10 years after surgery, which is correlated to their less symptoms of dysphagia. Another explanation is related to poor functional emptying of the WS reconstruction due to the inferior mechanical emptying compared to a NGT.
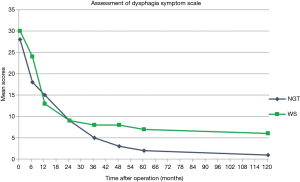
The result of our study showed that patients with esophageal cancer may obtain survival benefits from NGT reconstruction. This benefit may start early (2 years after surgery) (12), become steady (5 years after surgery) (13) and show significance (10 years after surgery). Esophagectomy is a highly invasive procedure with relatively poor prognosis. Surgical resection and lymph node dissection remain the most significant and effective methods in the treatment of esophageal cancer. Factors influencing long-term survival had not been attracting great attentions because of poor survival. Gastric tube may be a better alternative choice for better HRQL (13), but its influence on survival has long been controversial. It has been proven that survival diminished obviously with increasing number of regional lymph nodes involved (1). Anatomic differences between gastric tube reconstruction and WS reconstruction are that the lesser curvature of the stomach and the cardia can be resected in the gastric tube reconstruction, thus more lymph nodes and lymphatic networks could be harvested. This may reduce local recurrence and metastasis, contributing to the survival benefit.
Conclusions
Because of a significantly better long-term survival (P=0.027), gastric tube reconstruction may be a better choice for patients undergoing oncologic esophagectomy. HRQL recovers to a level comparable in most patients who survive 10 years after esophagectomy for cancer, although some digestive tract symptoms, like dysphagia, may last during the whole postoperative period and represent a poor prognosis.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All patients signed the consent, and the ethics committee approved this study.
References
- Rice TW, Rusch VW, Apperson-Hansen C, et al. Worldwide esophageal cancer collaboration. Dis Esophagus 2009;22:1-8. [Crossref] [PubMed]
- Derogar M, Orsini N, Sadr-Azodi O, et al. Influence of major postoperative complications on health-related quality of life among long-term survivors of esophageal cancer surgery. J Clin Oncol 2012;30:1615-9. [Crossref] [PubMed]
- Rouvelas I, Zeng W, Lindblad M, et al. Survival after surgery for oesophageal cancer: a population-based study. Lancet Oncol 2005;6:864-70. [Crossref] [PubMed]
- Daly JM, Fry WA, Little AG, et al. Esophageal cancer: Results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg 2000;190:562-72; discussion 572-3. [Crossref] [PubMed]
- Lagergren P, Avery KN, Hughes R, et al. Health related quality of life among patients cured by surgery for esophageal cancer. Cancer 2007;110:686-93. [Crossref] [PubMed]
- Derogar M, Lagergren P. Health-related quality of life among 5-year survivors of esophageal cancer surgery: a prospective population-based study. J Clin Oncol 2012;30:413-8. [Crossref] [PubMed]
- Akiyama H, Miyazono H, Tsurumaru M, et al. Use of the stomach as an esophageal substitute. Ann Surg 1978;188:606-10. [Crossref] [PubMed]
- Boone J, Livestro DP, Elias SG, et al. International survey on esophageal cancer: part I surgical techniques. Dis Esophagus 2009;22:195-202. [Crossref] [PubMed]
- Ndoye JM, Dia A, Ndiaye A, et al. Arteriography of three models of gastric oesophagoplasty: the whole stomach, a wide gastric tube and a narrow gastric tube. Surg Radiol Anat 2006;28:429-37. [Crossref] [PubMed]
- Poghosyan T, Gaujoux S, Chirica M, et al. Functional disorders and quality of life after esophagectomy and gastric tube reconstruction for cancer. J Visc Surg 148:e327-35. [Crossref] [PubMed]
- Zhang C, Wu QC, Hou PY, et al. Impact of the method of reconstruction after oncologic oesophagectomy on quality of life–a prospective, randomized study. Eur J Cardiothorac Surg 2011;39:109-14. [Crossref] [PubMed]
- Zhang M, Wu QC, Li Q, et al. Comparison of the health-related quality of life in patients with narrow gastric tube and whole stomach reconstruction after oncologic esophagectomy: a prospective randomized study. Scand J Surg 2013;102:77-82. [Crossref] [PubMed]
- Zhang M, Li Q, Tie HT, et al. Methods of reconstruction after esophagectomy on long-term health-related quality of life: a prospective, randomized study of 5-year follow-up. Med Oncol 2015;32:122. [Crossref] [PubMed]


