Hedinger syndrome: first experience and two-year follow-up in patients with carcinoid heart disease
Introduction
Neuroendocrine tumours (NET) are rare, ubiquitous neoplasms that may occur anywhere in the human body. Their incidence is increasing and currently estimated at 6.98 per 100,000 population (1). NETs arise from cells of the diffuse neuroendocrine system and are characterized by their ability to produce peptide hormones, biogenic amines and other vasoactive substances. A common feature of all NETs is their ability to metastasize (2). They arise frequently within the gastro-entero-pancreatic system (GEP-NET), especially in the terminal ileum, and grow slowly over years. These tumors were originally termed “carcinoids”. Commonly they cause only few symptoms until they are large or have metastasized. Approximately 20% of GEP-NET patients present with symptoms of carcinoid syndrome (CS) mainly characterized by seizure-like vasomotor changes (flushing, hypotension), diarrhea, and/or bronchospasm (3). Once metastasized, vasoactive substances released by the tumor exert systemic effects if they bypass portal circulation and escape hepatic metabolism. However, about 5% of patients, particularly those with primary pulmonary sites or retroperitoneal metastases, may present with CS without liver metastases (4).
Carcinoid heart disease (CHD), which is characterized by the development of plaque-like, fibrous endocardial thickening and retraction involve mainly the right-sided heart valves, as the most serious complication of CS and is known as Hedinger’s disease. These alterations can be detected in about half of all CS patients and are associated with significantly increased morbidity and mortality (5). The pathophysiology of CHD is poorly understood; chronic exposure of the endocardium to elevated peptide hormones, especially serotonin [5-hydroxytryptamine] is considered as one of the key factors. Functionally, the tricuspid valve is affected by regurgitation, whereas the pulmonary valve usually exhibits both regurgitation and stenosis, leading to severe right heart failure (6). We report our primary experience on 6 patients with CHD after implementation of a multidisciplinary, standardized treatment protocol.
Methods
The present study was a non-randomized, single-center retrospective study including six consecutive high-risk patients who underwent multi-valvular surgery caused by CHD at our department between July 2015 and October 2018. Patient and operative demographics were recorded in a prospective institutional database and retrospectively extracted and evaluated. Survival was obtained by active follow-up.
Perioperative management
Based on the recommendations of an interdisciplinary tumor-board, the indication for surgery was re-evaluated by a team involving cardiac surgeons, endocrinologists, cardiologists and anesthetists. Once planned for surgery, a standardized medical regime was established for all patients. All subjects were started on an octreotide (Novartis, Basel, Switzerland) infusion of 100 mcg/h, 12 h prior to surgery and continued for 48 h after surgery. After this period, patients were started on subcutaneous octreotide 200 mcg three times a day for the next 14 days. Thereafter, patients were recommenced on their long-acting somatostatin analogue.
For the treatment of a carcinoid crisis (tachycardia, bronchospasm, flushing and labile blood pressure), intravenous bolus applications of octreotide 100 mcg were added to the continuous infusion, as well as bolus infusions of steroids and antihistamines. As all patients presented with pulmonary hypertension, inhaled nitric oxide therapy combined with iloprost inhalation was started intraoperatively after weaning from CPB, and was continued until weaning from the ventilator.
Surgical management
Surgery was performed in 4 patients presenting with multivalvular lesions, by median sternotomy, standard cardiopulmonary bypass (CPB) technique with ascending aortic and double venous cannulation, mild hypothermia and cold crystalloid cardioplegic arrest. In the other 2 patients presenting only with isolated tricuspid valve regurgitation, surgery was performed by a right-sided anterolateral endoscopic approach through the 4th intercostal space with double-lumen endotracheal intubation. These two patients were operated on the normothermic beating-heart. A histological sample of the pericardium/endocardium was taken in all patients.
Intraoperative echocardiography
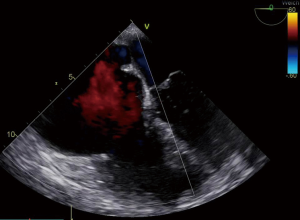
Transesophageal echocardiography (TOE) was performed prior to CPB and immediately after decannulation in all patients. TOE was performed with a multiplane 2.9–6.7 MHz (6T-RS) phased-array-probe (Vivid I, GE Healthcare, Milwaukee, WI, USA). Figure 1 shows an echocardiographic finding of severely fibrosed and fixed tricuspid valve before CPB.
Results
Patients
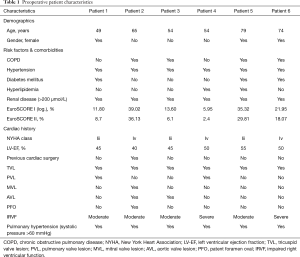
Six consecutive patients (three females) with Hedinger syndrome presenting with multivalvular lesions were enrolled in the study. Mean age was 63±12 years (range, 49 to 79 years). Detailed baseline patient characteristics are listed in Table 1. All patients had signs and symptoms of right-sided heart failure including pulmonary hypertension. The mean logistic EuroSCORE I was 21.95%±13.4% & EuroSCORE II was 17.2%±14.2%, and all patients were in NYHA functional class III–IV. In all patients, the tricuspid valve was involved showing severe regurgitation or a combined lesion. Table 2 exemplifies the intraoperative echo findings prior to CPB.
Full table

Full table
Surgery
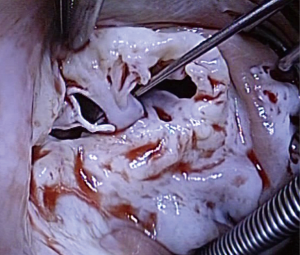
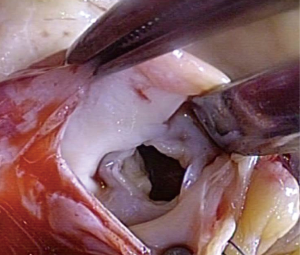
Tricuspid valve repair was possible in 4 patients, whereas 2 needed replacement as these two patients showed severe fibrosis of the tricuspid valve. An example of a completely destroyed tricuspid valve is given in Figures 2,3. In two patients (#5 and #6), the operation was performed endoscopically on the beating heart, under normothermia without cross-clamping. The pulmonary valve was concomitantly replaced in 2 patients with additional bovine pericardial patch enlargement of the pulmonary artery. In 2 patients, the aortic valve was replaced. One patient has got mitral valve repair using annuloplasty device. Mean CPB-time was 131±66 min. and aortic cross-clamping time was 61±50 min (Table 3). Patient #6 needed central veno-arterial ECMO support on the first postoperative day, however, this patient died 3 days later due to massive neuroendocrine enzyme storm (carcinoid crisis). This patient received an endoscopic implantation of a 31 mm Hancock II prosthesis (Medtronic Inc., Minneapolis, MN, USA) into the preserved insufficient native tricuspid valve with complete preservation of the subvalvular apparatus.

Full table
Histological examination
All biopsies showed different degrees of fibrotic connective tissues disorders of the myocardium, as well as interstitial fibroblast infiltration. Especially in patient #6, severe endocardial fibrosis and plaque-like deposits composed of myofibroblasts, extracellular matrix components (collagen and myxoid ground substance) were also diagnosed.
Follow-up
At 13 months postoperatively, one patient showed severe tricuspid stenosis and underwent uneventful re-operation with replacement of the tricuspid valve. One patient died 18 months postoperatively related to the underlying tumour disease. At 30 months follow-up, the remaining 4 patients were alive and asymptotic (NYHA I).
Discussion
The present study describes a series of 6 patients presenting with the rare entity of CHD, who were operated at our center. As CHD represents a complex disease with known poor prognosis, a multidisciplinary team approach is essential. This peculiar symptom complex of metastatic carcinoids of the small intestine with severe valvular defects, especially in the right side of the heart, has been initially described 1953 by the pathologist Christoph Hedinger [1917–1999] in Zurich. He had had the chance to observe two females, both presenting with oedema, cachexia, hepatomegaly and cardiac decompensation. Both patients died due to cardiac decompensation and during autopsy, he diagnosed multiple carcinoids within the intestine combined with right-sided cardiac hypertrophy and constricted tricuspid valve tissue (7).
NETs, previously known as carcinoid tumors, are rare malignancies, with an incidence of 5.1/100,000/year, occurring mainly in the gastrointestinal tract (67.5%) followed by the bronchopulmonary system (25.3%) (8,9). CHD is diagnosed approximately 1.5 years after the initial diagnosis of NET and CS (10). However, diagnosis at the earliest stage is essential, as the development of right ventricular dysfunction portends a poor prognosis and represents a major cause of morbidity and mortality. In untreated patients, cardiac decompensation will result in mortality rates up to 43% (11-14).
Patients with the rare diagnosis of CHD presenting with metastatic NETs and CHD are patients at highest risk. Such patients should be treated in specialized centers by a multidisciplinary team including endocrinologists, oncologists, cardiologists, pathologists, and surgeons with experience in the treatment of this complex entity (15). Without a timely intervention, NET patients with CHD will definitively develop progressive right heart failure in parallel with a significant decrease in their life expectancy compared with those NET patients without CHD (5).
Compared to the right side of the heart, the left-sided heart is rarely affected, due to the pulmonary metabolism, and deactivation of hormonal substances (5). Nevertheless, left-sided involvement might also be present in patients presenting a patent foramen oval (16), which appears to be more common in patients with CS and CHD compared to the general population (17). In this regard, it is a clinical challenge to distinguish symptoms of otherwise right-sided heart failure to those from end-stage metastatic carcinoid disease, as both can present with progressive fatigue, oedema, and ascites (18). In our patient group right heart failure was present in all patients with severe tricuspid valve regurgitation and pulmonary hypertension. Moreover, we observed severe fibrosis of the endocardium, which in turn lead to right-sided heart failure. An unusual observation was made in patient number 6, this showed a severe carcinoid crisis 24 hours after the implantation of a V-A ECMO. The interdisciplinary analysis of such phenomenon implies that through the ECMO implantation the pulmonary metabolism was bypassed, which was the only barrier to protect the systemic circulation against the vasoactive substances, which consequently triggered a severe carcinoid crisis. Therefore, V-A-ECMO in such patients as a support of right sided heart failure in the future should be avoided.
In our series, the pulmonary valve was involved in 2 patients. In these two patients, in addition to pulmonary valve replacement, the fibrosed pulmonary artery was enlarged by a pericardial bovine patch. Moreover, the left-sided heart was involved in 2 patients; one patient presented with a patent foramen oval and the other showed a lung metastasis of the NET, also releasing serotonin into the left-sided circulation.
In our small series of 6 patients, who were treated within a 2.5-year time interval, 30-day mortality was 16.7% (1/6 patients). Another patient died 18 months postoperatively. In this picture in regards to perioperative mortality, it must be stated however, that late survival in these patients is poor. Surgery improves outcomes, but the reported perioperative mortality is high (17%, range: 1–63%) (19). Complications accountable for the elevated mortality rate are right-sided heart failure, coagulopathy and carcinoid crisis (20). Only few reports have been published on selected surgical techniques for CHD. Our results are comparable to Connolly et al., who published the so far largest series of patients operated with CHD. Over a 27-year period, they retrospectively analysed a series of 195 consecutive patients showing 10% perioperative deaths in their early period. After 2000, they were able to reduce perioperative mortality down to 6%. Moreover, they could show survival rates at 1, 5 and 10 years of 69%, 35% and 24%, respectively (18). Although mortality rates are high and survival is much worse than in conventional valve surgery, without operation, only 10% of patients with Hedinger syndrome will survive 2 years after onset of symptoms (21).
Conclusions
These data may raise awareness on Hedinger syndrome (CHD), which is a rare and severe manifestation of advanced NETs and is associated with increased morbidity and mortality related to (right-sided) heart failure. The underlying mechanisms and pathophysiology in the development of CHD remains obscure, despite the emerging evidence that serotonin plays a major role in the process of valve destruction and myocardial dysfunction. The implantation of veno-arterial ECMO as a support for heart failure should be critically examined. Early detection of cardiac involvement is of utmost importance to offer surgical treatment options for these patients as prognosis of patients with CHD is poor if left untreated. Finally, the management of patients with CHD is extremely complex and requires a multidisciplinary approach involving specialists with broad experience in the field.
Limitations
The present study was performed at single tertiary care medical center including a multidisciplinary team approach. Hence, the generalizability of our findings may not extend to all centers worldwide. A basic limitation of our study is the small sample size of patients presenting with Hedinger syndrome, however it represents our first experience in treating this very high-risk group of patients. According to the current situation in Germany, one can assume that many patients at high or highest risk presenting with CHD, were not referred to surgery.
Acknowledgments
The authors are sincerely grateful to Gina Spangel, Andreas Sander and Wolfgang Ristau (Institute of Quality Controlling, West German Heart and Vascular Centre Essen) for their enormous efforts and support in data acquisition to finish this work.
Footnote
Conflicts of Interest: The study has been presented at the 68th international congress of the European Society for Cardiovascular and Endovascular Surgery, on 22–24 May 2019 in Groningen, Netherlands.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study did not require an approval as the study included a small number of subjects and data analysis and interpretation has been retrospectively and anonymously performed as indicated by our institutional review board and preapproved from all patients presented for surgery in our institution.
References
- Dasari A, Shen C, Halperin D, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol 2017;3:1335-42. [Crossref] [PubMed]
- Kloppel G. Classification and pathology of gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer 2011;18 Suppl 1:S1-16. [Crossref] [PubMed]
- Halperin DM, Shen C, Dasari A, et al. Frequency of carcinoid syndrome at neuroendocrine tumour diagnosis: a population-based study. Lancet Oncol 2017;18:525-34. [Crossref] [PubMed]
- Pape UF, Perren A, Niederle B, et al. ENETS Consensus Guidelines for the management of patients with neuroendocrine neoplasms from the jejuno-ileum and the appendix including goblet cell carcinomas. Neuroendocrinology 2012;95:135-56. [Crossref] [PubMed]
- Pellikka PA, Tajik AJ, Khandheria BK, et al. Carcinoid heart disease. Clinical and echocardiographic spectrum in 74 patients. Circulation 1993;87:1188-96. [Crossref] [PubMed]
- Grozinsky-Glasberg S, Grossman AB, Gross DJ. Carcinoid Heart Disease: From Pathophysiology to Treatment--'Something in the Way It Moves'. Neuroendocrinology 2015;101:263-73. [Crossref] [PubMed]
- Isler P, Hedinger C. Metastatic carcinoid of the small intestine with severe valvular defects especially in the right part of the heart and with pulmonary stenosis; a peculiar symptom complex. Schweiz Med Wochenschr 1953;83:4-7. [PubMed]
- Solcia E, Rindi G, Paolotti D, et al. Clinicopathological profile as a basis for classification of the endocrine tumours of the gastroenteropancreatic tract. Ann Oncol 1999;10 Suppl 2:S9-15. [Crossref] [PubMed]
- Rindi G, Villanacci V, Ubiali A. Biological and molecular aspects of gastroenteropancreatic neuroendocrine tumors. Digestion 2000;62 Suppl 1:19-26. [Crossref] [PubMed]
- Moller JE, Pellikka PA, Bernheim AM, et al. Prognosis of carcinoid heart disease: analysis of 200 cases over two decades. Circulation 2005;112:3320-7. [Crossref] [PubMed]
- Lundin L, Norheim I, Landelius J, et al. Carcinoid heart disease: relationship of circulating vasoactive substances to ultrasound-detectable cardiac abnormalities. Circulation 1988;77:264-9. [Crossref] [PubMed]
- Ross EM, Roberts WC. The carcinoid syndrome: comparison of 21 necropsy subjects with carcinoid heart disease to 15 necropsy subjects without carcinoid heart disease. Am J Med 1985;79:339-54. [Crossref] [PubMed]
- Moyssakis IE, Rallidis LS, Guida GF, et al. Incidence and evolution of carcinoid syndrome in the heart. J Heart Valve Dis 1997;6:625-30. [PubMed]
- Connolly HM, Schaff HV, Mullany CJ, et al. Carcinoid heart disease: impact of pulmonary valve replacement in right ventricular function and remodeling. Circulation 2002;106:I51-6. [PubMed]
- Dobson R, Burgess MI, Pritchard DM, et al. The clinical presentation and management of carcinoid heart disease. Int J Cardiol 2014;173:29-32. [Crossref] [PubMed]
- Mansencal N, Mitry E, Forissier JF, et al. Assessment of patent foramen ovale in carcinoid heart disease. Am Heart J 2006;151:1129.e1-6. [Crossref] [PubMed]
- Mansencal N, Mitry E, Pilliere R, et al. Prevalence of patent foramen ovale and usefulness of percutaneous closure device in carcinoid heart disease. Am J Cardiol 2008;101:1035-8. [Crossref] [PubMed]
- Connolly HM, Schaff HV, Abel MD, et al. Early and Late Outcomes of Surgical Treatment in Carcinoid Heart Disease. J Am Coll Cardiol 2015;66:2189-96. [Crossref] [PubMed]
- Manoly I, McAnelly SL, Sriskandarajah S, et al. Prognosis of patients with carcinoid heart disease after valvular surgery. Interact Cardiovasc Thorac Surg 2014;19:302-5. [Crossref] [PubMed]
- Bhattacharyya S, Raja SG, Toumpanakis C, et al. Outcomes, risks and complications of cardiac surgery for carcinoid heart disease. Eur J Cardiothorac Surg 2011;40:168-72. [Crossref] [PubMed]
- Connolly HM, Nishimura RA, Smith HC, et al. Outcome of cardiac surgery for carcinoid heart disease. J Am Coll Cardiol 1995;25:410-6. [Crossref] [PubMed]