
Lipoxin A4 ameliorates alveolar fluid clearance disturbance in lipopolysaccharide-induced lung injury via aquaporin 5 and MAPK signaling pathway
Introduction
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS), is one of the dominant causes of the high mortality of critically ill patients (1,2), companied by the increased permeability of protein and an alteration of the capacity of alveolar fluid clearance (AFC) associated with increased mortality (3-5). AFC capacity refers to the capacity of the alveolar epithelial membrane to remove water from distal air spaces in the lung (4,6,7). Studies have shown that AFC contributes to providing an appropriate alveolar air-liquid interface essential for adequate gas blood exchange and host barrier defense against exogenous pathogen (4,5). Aquaporin (AQP)-type water channels are essential components in AFC maintenance (8-10). Among the different AQPs, AQP5 is especially expressed in apical membrane of serous cells in submucosal glands and alveolar type I epithelial cells in the lungs, playing important roles in osmotic water permeability of the alveolar-capillary barrier (11). It is found that the stimulation of lipopolysaccharide (LPS) augmented the water permeability of lung epithelial cells by changing the cell surface AQP5 expression and the distribution (11).
Lipoxins are endogenous lipids involving in the resolution of inflammation, including inhibiting pro-inflammatory cytokine release, decreasing leukocyte-mediated injury, and stimulating macrophage efferocytosis (12,13). Lipoxin A4 (LXA4) is a member of lipoxin family that functions as “braking signals” in the inflammatory response and switches the inflammatory response to the resolution phase (13-15). LXA4 has been proved to present the distinct pro-resolution and anti-edema properties in an ALI model (16). In one study, LXA4 played a strong protective role in ALI, in part by remaining the E-cadherin expression and airway epithelial permeability (17). Thus, we supposed that AQP5, which is especially located in the alveolar type I epithelial cells and functioning as maintaining the fluid balance, might be related to the protective role of LXA4 since AQP5 helped to preserve the airway epithelial permeability, as the further exploration of the mechanism of LXA4. However, it is reported that in the acute pancreatitis induced ALI model, LXA4 plays the anti-inflammatory (ALR) effect by attenuating endothelial cell growth inhibition and apoptosis, regulating the AQP-5 expression to stabilize endothelial cell and reducing alveolar fluid exudation (18). Thus, whether AQP5 is involved in the protective role of LXA4 in the airway epithelial permeability maintenance is still unknown yet. This study was aimed to explore, in a ALI model built by LPS stimulation, whether AFC-protective effect of LXA4 in ALI is regulated by AQP5 and its potential cell signal pathway, with the aim of elucidating the possible mechanism involved in the effect of LXA4 on ALI.
Methods
Animals and preparation
The animal experiments in our study were authorized by the Animal Care and Use Ethics Committee of our institution (KT2018061). The experiments were carried out according to the guide and instruction of the Care and Use of Laboratory Animals. Healthy male C57BL/6 mice (20–30 g, 6–8 weeks old) were purchased from China Medical University. The mice were housed in a specific pathogen-free (SPF) environment on a 12 h light/12 h dark cycle. The operations were performed under anesthesia.
Experimental mice were divided into four experimental groups (n=15 per group) randomly: (I) control group; (II) LPS-induced ALI group (ALI group); (III) LXA4 group; and (IV) ALI + LXA4 group. ALI was induced by 50 µL of LPS (8 mg/kg) intraperitoneal (i.p.) administration as described previously (18). The sterilized phosphate buffered saline (PBS) worked as the solution of LPS before use. The equivalent amount of PBS was administered to the control group and LXA4 group. Eight hours after LPS or PBS instillation, LXA4 (0.1 mg/kg, i.p.) was administered as outlined in a previously published article for the two LXA4 groups (18). Mice were sacrificed by exsanguination and lungs were sampled 24 hours after the LPS or PBS administration. For five mice of each group, the lung wet/dry weight ratio was measured as described previously (19), and the same five lungs were used for the measurement of AFC. For the other mice of each group, the left lobes were paraffin-embedded for five mice, while the left lungs of the remaining five mice were instilled with 0.5 mL PBS four times for the bronchoalveolar lavage fluid (BALF), while the other parts of the lungs were stored at −80 °C. BALF was centrifuged for 10 min at 1,000 rev/min at 4 °C, and the supernatants of BALF were kept at −80 °C for the measurement of inflammatory cytokines.
AFC measurement
The measurement of AFC was according to the method described previously (19). After 24 h of LPS or PBS administration, under the anesthesia, the mouse was intubated with a tube through a tracheostomy. The left lung was excised to measure the ratio of lung wet to dry lung weight. Then, the trachea, right lung, and heart were excised en bloc to measure AFC. A 37 °C solution made from saline containing 5% albumin and 0.15 mg/mL Evans blue dye was injected slowly into the right lung. To make all of the instilled solution into the alveolar spaces, 12 mL/kg oxygen was delivered after the solution, and then inflated with 100% oxygen with the 5 cm H2O airway pressure in an incubator at 37 °C. AFC was calculated according to the following formula (19-21):
AFC =[(Vi-Vf)/Vf]×100, where Vf=(Vi ×EBi)/EBf; Vi is the volume of instilled solution made from saline and albumin, and Vf is the volume of final alveolar fluid, which is given by where EB is the concentration of Evans blue dye in instilled solution (i) and in the final alveolar fluid (f).
Morphological changes
For histological examination, left lung tissues were processed with 4% paraformaldehyde for 24 h, embedded in paraffin wax, and then sectioned into 5 µm thicknesses. After the staining of hematoxylin and eosin, the slides were examined with a light microscope (Olympus, Tokyo, Japan) and analyzed under the blinded observation to evaluate the ALI severity by the following variables: (I) alveolar hemorrhage; (II) alveolar congestion; (III) alveolar wall thickness or formation of hyaline membrane; and (IV) inflammatory infiltration or aggregation of neutrophils in alveoli or vessel wall. For each item, the degree was graded from 0 to 4 for as described previously (22). The lung injury score (total score, 0–16) were calculated by the summarization of the four variables.
Measurement of interleukin (IL)-6 and tumor necrosis factor (TNF)-α in BALF
The levels of TNF-α, and IL-6 in BALF were measured using ELISA kits (San Diego, Biolegend, CA, USA) in accordance with the manufacturer’s protocols.
Myeloperoxidase (MPO) activity of lung homogenate
To measure MPO enzymatic activity, the lung tissues were homogenized in 50 mmol/L PBS containing 0.5% hexadecylammonium bromide and 5 mmol/L EDTA (pH 6.0). The lung homogenate was centrifugated at 12,500×g for 20 min at 4 °C. After incubated in 50 mmol/L PBS containing 30% H2O2 and o-dianisidinedihyddrochloride (167 µg/mL, Sigma-Aldrich, St Louis, MA, USA), the supernatant fluid was prepared for the detection of the absorbance at 460 nm over 3 min spectrophotometrically represented the enzymatic activity of MPO (23).
Immunohistochemistry (IHC) staining
After paraffin removal in xylene, rehydration, antigen retrieval in a pressure cooker with citrate buffer, pH 6.0, for 1 min after reaching boiling temperature to, and endogenous peroxidase quenching with 3% H2O2 for 15 min, slides were incubated overnight at 4 °C with AQP5 primary antibody (1:100 dilution, Abcam, Cambridge, UK) or with pre-immune serum as a negative control. Subsequently after the incubation with biotinylated secondary antibody for 1 h at room temperature, and finally with peroxidase-conjugated streptavidin, using diaminobenzidine as the substrate. The slides were stained with hematoxylin for 30 sec. The slides were observed under a microscope and photographed (Olympus, Tokyo, Japan).
Immunoblotting analysis
Immunoblotting analysis of frozen lung homogenates was processed as previously described (24). Equal amounts of protein were loaded in each lane. The protein were separated by 10% SDS-polyacrylamide gel electrophoresis, and then transferred to polyvinylidene difluoride (PVDF) membranes. The PVDF membranes were blocked for 2 h with 5% skimmed milk, then, incubated with the primary antibodies of AQP5 (Bioss antibiodies, Beijing, China), p-p38 (Cell Signaling Technology, MA, USA), p38 (Cell Signaling Technology, MA, USA), p-JNK (Cell Signaling Technology, MA, USA), JNK (Cell Signaling Technology, MA, USA) and β-actin (Cell Signaling Technology, MA, USA) at dilutions of 1:1,000 or 1:2,000 and incubated overnight at 4 °C, and secondary antibodies (Anti-rabbit IgG, Cell Signaling Technology, MA, USA) at dilution of 1:2,000. The signals were detected using enhanced chemiluminescence (GE Healthcare UK Ltd, Gloucester, UK), and the protein bands were analyzed by densitometry (Metamorph/Evolution MP 5.0/B X51) with β-actin as a loading control.
Statistical analysis
All values were reported as mean ± standard error of the mean (SEM). Data were statistically analyzed by one-way ANOVA, followed by a Newman-Keuls post hoc test. P<0.05 was defined as statistically significant. The above analysis and graphs were performed using GraphPad Prism 5.0 (GraphPad, San Diego, CA, USA).
Results
LXA4 improved the survival rate in LPS-induced ALI
As shown in Figure 1A, LXA4 treatment of mice in an ALI model induced by LPS challenge increased the survival rate of 7 days from 40% to 65% of animals (P<0.01), while the survival rate of 7 days in the control group was 100%. Thus, LXA4 reduces death in the ALI model induced by LPS stimulation.
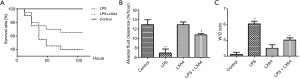
LXA4 restored AFC capacity reduced by LPS stimulation while decreasing the lung wet-to-dry ratio by reducing lung fluids
The AFC was decreased significantly by LPS stimulation in mice, while the administration of LXA4 partially restored the AFC, although LXA4 could not restore it to the level of the control group. When comparing the wet-to-dry lung ratio in all groups, in accordance with the AFC capacity, the LPS-induced ALI group had a high ratio due to increased lung fluids and the impaired capacity of AFC. LXA4 treatment led to a decrease in the lung wet-to-dry ratio by decreasing lung fluids and partially increasing the AFC (Figure 1B,C).
LXA4 alleviated lung inflammatory response and lung injury induced by LPS stimulation
The count of total cell and percentage of neutrophils in BALF were used as markers of acute lung inflammation. In the ALI model group, the total cell count and percentage of neutrophils in BALF were markedly increased due to LPS stimulation (Figure 2A,B). Since MPO is a marker of neutrophil infiltration in the lung, we evaluated the MPO concentration in lung homogenates (Figure 2C). Consistent with the inflammatory cell response in BALF, the MPO concentration in the lung homogenate was markedly increased in the ALI group. LXA4 treatment in LPS-induced ALI alleviated lung inflammation by reducing the absolute cell count in BALF and neutrophil infiltration as represented by the BALF neutrophil percentage and the MPO concentration in the lung homogenate.

As the inflammatory indicators in the lung, concentrations of the proinflammatory cytokines in BALF, TNF-α and IL-6, were evaluated (Figure 2D,E). An LPS injection led to an increase in TNF-α as well as IL-6 concentrations in BALF. Treatment with LXA4 after LPS administration helped in a partial recovery from inflammatory injury by reducing TNF-α and IL-6 concentrations in BALF.
As a result of the inflammatory response in ALI, a pathological examination was performed to evaluate morphological damage. As shown in Figure 2 (Figure 2G,H,I,J), the control group showed a normal lung structure, while LPS stimulation led to inflammatory cell congestion, alveolar wall thickening and alveolar hemorrhage. For several severe cases, a hyaline membrane could also be seen. When using an ALI score to quantify and compare ALI between different groups (Figure 2F), the LPS-induced ALI group presented with an evident ALI, while LXA4 treatment markedly ameliorated lung injury.
Thus, LXA4 alleviated LPS-induced lung injury as demonstrated by a reduced cell count and neutrophil infiltration, and decreased the concentrations of the inflammatory cytokines, TNF-α, and IL-6 in BALF, and the enzymatic activity of MPO in the tissue of lungs with reduced morphological damage as well.
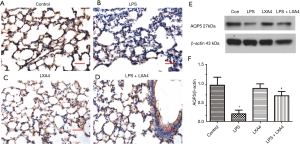
LXA4 treatment prevented LPS-induced AQP5 down-regulation in an ALI model
Since it is known that the AQP5 water channel modulated AFC in lungs, we designed to explore the effect of LXA4 on AQP5. As shown in Figure 3, AQP5 was decreased by LPS stimulation. LXA4 raised the expression of AQP5 in the lungs as determined by IHC staining and western blotting. Thus, LXA4 treatment in a ALI model induced by LPS challenge led to increased AQP5 expression. As shown in IHC staining, AQP5 expression increased markedly in epithelial cells of alveoli.
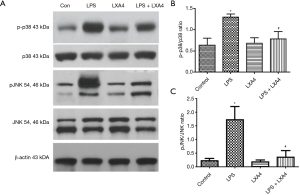
A protective role for LXA4 in the ALI model via the decreased phosphorylation p38 and JNK pathways
To explore the potential cell signaling pathways involved in the protective role of LXA4 in an ALI model induced by LPS, we investigated the phosphorylation of p38 and JNK. In LPS challenge group, p38 and JNK were markedly phosphorylated more to activate the inflammatory pathway. LXA4 treatment partially decreased the phosphorylation level of p38 and JNK (Figure 4), indicating the potential cell signaling pathway of LXA4 in ALI by the downregulation of p38 and JNK in a ALI model induced by LPS.
Discussion
To determine the barrier disturbance of fluid transport in ALI, we employ a LPS-induced ALI model in mice. We demonstrated the accumulation of water in the lungs measured as the wet-to-dry lung ratio increase and AFC decline, as well as the increase in lung pro-inflammatory cytokines in BALF and lung injury severity in morphology evaluation. After LXA4 treatment, the impairment of fluid transport was partially recovered and was accompanied by a decrease in the inflammatory response as well as reduced morphological lung injury changes in response to LPS. These phenomena correlated with an improvement in the survival rate in the LPS + LXA4 group when compared to the untreated control group. In accordance with previous studies on the effect of LXA4 in acute pancreatitis-associated ALI (18), our study confirmed the protective role of LXA4 in ALI via the inhibition of inflammation and the restoration of AFC function. In the two aforementioned studies, the authors found a protective role for LXA4 via mechanisms involving either the cytoskeleton protein, F-actin (18), or the AJs marker, E-cadherin (17), which both work to maintain the integrity of barrier function. In our study, similar to the authors’ observations, we found partially restored AFC function with LXA4 treatment, also as a result of the maintenance of the integrity of barrier function.
AQPs facilitate transcellular water flux across the epithelium of alveoli (25). Among the thirteen AQPs in mammals (25), AQP5, which especially located and expressed in the pulmonary capillary endothelial cells and type I epithelial cells, is one of the most important AQPs for water transport (10). AQP5 influenced osmotic transendothelial/transepithelial water permeability and the knockout of AQP5 even decreased the water permeability to 10-fold (26). However, participation of different AQPs on different localization was reported to participate into different types of lung injury (9). For example, in the ALI caused by direct injury to epithelium such as acid aspiration and mechanical ventilation, AQP4, targeted primarily the alveolar epithelium, decreased in the lung tissue, while AQP5, expressed both in the capillary endothelium and alveolar epithelium, decreased in a model such as endotoxemia induced by LPS (9). Although the LPS-induced ALI model has been proved to primarily target the capillary endothelium as the result of the systemic inflammatory response of the body (27), many studies showed that in LPS-induced ALI, the endothelium response is less apparent than that of epithelial cells, with AQP5 expression decreasing markedly (9,28), suggesting AQP5 as a marker of the integrity of the epithelium barrier. In another model of ALI by Pseudomonas aeruginosa, depletion of AQP5 has also been shown to exacerbate the lung injury by impairing the function of the epithelium barrier, and promoting bacterial blood dissemination during infection (29). In our study, by IHC staining, the AQP5 expression was increased in the epithelium of alveoli, thus, based on its protective role the mechanism described above, LXA4 protected AFC capacity by increasing the expression of AQP5 and enhanced the epithelium barrier function. In addition to our study, another study on the protective role of LXA4 in ALI found that LXA4 helped to maintain barrier function by regulating E-cadherin expression. E-cadherin, as the critical component of cytoskeleton, help to stabilize the airway permeability by maintaining the stability of adherens junctions (17). The increased expression of E-cadherin by the treatment of LXA4 also suggested the protective role of LXA4 in the epithelium barrier function by stabilizing the epithelial adherens junctions.
But how AQP5 is regulated by the administration of LXA4 is still unknown as yet. In the current study, we found that LPS insult led to the phosphorylation of p38 and JNK and thus the activation of an inflammatory response and the impairment of AFC. LXA4 inhibited the phosphorylation of p38 and JNK and partially reduced the inflammatory response and the extravasation of alveolar fluid. In a previous study on the protective role of LXA4, this was found to exert a protective role being anti-apoptotic in acute pancreatitis-associated ALI (18). In another study using BML-111, a LXA4 receptor agonist, its prophylactic significance in ALI was observed by targeting mitogen-activated protein kinase (MAPK) signaling but not the mammalian target of rapamycin pathway. Meanwhile, the authors found that BML-111 stimulated autophagy in alveolar macrophages, attenuated the LPS-induced cell apoptosis of alveolar macrophages, and promoted the resolution of ALI (30). Based on these results, we hypothesized that LXA4 may play a protective role in LPS-induced ALI via the MAPK pathway, which is possibly related to an anti-apoptotic mechanism in the inflammatory cascade. In ALI, LPS treatment activated p38 MAPK and JNK via phosphorylation in the lungs, relating to the downregulation of AQP5. The corresponding kinase inhibitor could inhibit the LPS-induced downregulation of AQP5, indicating the relationship of p38 and JNK with AQP5 expression (31). However, LXA4 interacts with several receptors, such as ALR, formyl peptide receptor 2, and formyl peptide receptor 3, to exert its downstream protective effects (32) by the pathway involving protein kinase C/Src suppressed C kinase substrate/F-actin, phosphoinositide 3-kinase, nuclear factor-kappa B, protein kinase B, Nuclear factor-like 2 protein (17,18,33) and other pathways involved in TNF-α- or LPS-induced alveolar fluid barrier dysfunction and pulmonary edema (16,34). The crosstalk or interaction between such molecules still needs to be clarified in future.
Taken together, our current findings demonstrate that LXA4 plays a protective role in LPS-induced ALI through the restoration of AFC capacity and the upregulation of AQP5 expression in lung tissue by inhibiting the phosphorylation of p38 and JNK. These findings suggest potential new strategies for improving anti-inflammation therapy for the impairment of alveolar fluid transport in ALI.
Acknowledgments
Funding: This work was supported by National Natural Science Foundation of China (NSFC-81700041) (Y Yin) and Liaoning Science and Technology Project (2018011494-301) (X Zhou, and X Gu).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The animal experiments in our study were authorized by the Animal Care and Use Ethics Committee of our institution (KT2018061).
References
- Sjoding MW, Hofer TP, Co I, et al. Interobserver Reliability of the Berlin ARDS Definition and Strategies to Improve the Reliability of ARDS Diagnosis. Chest 2018;153:361-7. [Crossref] [PubMed]
- Vadász I, Sznajder JI. Gas Exchange Disturbances Regulate Alveolar Fluid Clearance during Acute Lung Injury. Front Immunol 2017;8:757. [Crossref] [PubMed]
- Huppert LA, Matthay MA. Alveolar Fluid Clearance in Pathologically Relevant Conditions: In Vitro and In Vivo Models of Acute Respiratory Distress Syndrome. Front Immunol 2017;8:371. [Crossref] [PubMed]
- Matthay MA. Alveolar fluid clearance in patients with ARDS: does it make a difference? Chest 2002;122:340S-3S. [Crossref] [PubMed]
- Laffey JG, Matthay MA. Fifty Years of Research in ARDS. Cell-based Therapy for Acute Respiratory Distress Syndrome. Biology and Potential Therapeutic Value. Am J Respir Crit Care Med 2017;196:266-73. [Crossref] [PubMed]
- Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. N Engl J Med 2005;353:2788-96. [Crossref] [PubMed]
- Planès C, Leyvraz C, Uchida T, et al. In vitro and in vivo regulation of transepithelial lung alveolar sodium transport by serine proteases. Am J Physiol Lung Cell Mol Physiol 2005;288:L1099-109. [Crossref] [PubMed]
- Wang Q, Yan SF, Hao Y, et al. Specialized Pro-resolving Mediators Regulate Alveolar Fluid Clearance during Acute Respiratory Distress Syndrome. Chin Med J (Engl) 2018;131:982-9. [Crossref] [PubMed]
- Vassiliou AG, Manitsopoulos N, Kardara M, et al. Differential Expression of Aquaporins in Experimental Models of Acute Lung Injury. In vivo 2017;31:885-94. [PubMed]
- Song Y, Wang L, Wang J, et al. Aquaporins in Respiratory System. Adv Exp Med Biol 2017;969:115-22. [Crossref] [PubMed]
- Ohinata A, Nagai K, Nomura J, et al. Lipopolysaccharide changes the subcellular distribution of aquaporin 5 and increases plasma membrane water permeability in mouse lung epithelial cells. Biochem Biophys Res Commun 2005;326:521-6. [Crossref] [PubMed]
- Walker J, Dichter E, Lacorte G, et al. Lipoxin a4 increases survival by decreasing systemic inflammation and bacterial load in sepsis. Shock 2011;36:410-6. [Crossref] [PubMed]
- Wu B, Walker J, Spur B, et al. Effects of Lipoxin A4 on antimicrobial actions of neutrophils in sepsis. Prostaglandins Leukot Essent Fatty Acids 2015;94:55-64. [Crossref] [PubMed]
- Cheng Q, Wang Z, Ma R, et al. Lipoxin A4 protects against lipopolysaccharide-induced sepsis by promoting innate response activator B cells generation. Int Immunopharmacol 2016;39:229-35. [Crossref] [PubMed]
- Wang YP, Wu Y, Li LY, et al. Aspirin-triggered lipoxin A4 attenuates LPS-induced pro-inflammatory responses by inhibiting activation of NF-kappaB and MAPKs in BV-2 microglial cells. J Neuroinflammation 2011;8:95. [Crossref] [PubMed]
- Wang Q, Lian QQ, Li R, et al. Lipoxin A(4) activates alveolar epithelial sodium channel, Na,K-ATPase, and increases alveolar fluid clearance. Am J Respir Cell Mol Biol 2013;48:610-8. [Crossref] [PubMed]
- Cheng X, He S, Yuan J, et al. Lipoxin A4 attenuates LPS-induced mouse acute lung injury via Nrf2-mediated E-cadherin expression in airway epithelial cells. Free Radic Biol Med 2016;93:52-66. [Crossref] [PubMed]
- Shi Z, Ye W, Zhang J, et al. LipoxinA4 attenuates acute pancreatitis-associated acute lung injury by regulating AQP-5 and MMP-9 expression, anti-apoptosis and PKC/SSeCKS-mediated F-actin activation. Mol Immunol 2018;103:78-88. [Crossref] [PubMed]
- Wu XM, Wang HY, Li GF, et al. Dobutamine enhances alveolar fluid clearance in a rat model of acute lung injury. Lung 2009;187:225-31. [Crossref] [PubMed]
- Herrero R, Matute-Bello G. How to measure alterations in alveolar barrier function as a marker of lung injury. Curr Protoc Toxicol 2015;63:24.3.1-15.
- Gu X, Li P, Liu H, et al. The effect of influenza virus A on th1/th2 balance and alveolar fluid clearance in pregnant rats. Exp Lung Res 2011;37:445-51. [Crossref] [PubMed]
- Liu D, Zeng BX, Zhang SH, et al. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma agonist, reduces acute lung injury in endotoxemic rats. Crit Care Med 2005;33:2309-16. [Crossref] [PubMed]
- Rittirsch D, Flierl MA, Day DE, et al. Acute lung injury induced by lipopolysaccharide is independent of complement activation. J Immunol 2008;180:7664-72. [Crossref] [PubMed]
- Hou G, Yin Y, Han D, et al. Rosiglitazone attenuates the metalloprotease/anti-metalloprotease imbalance in emphysema induced by cigarette smoke: involvement of extracellular signal-regulated kinase and NFkappaB signaling. Int J Chron Obstruct Pulmon Dis 2015;10:715-24. [PubMed]
- Wittekindt OH, Dietl P. Aquaporins in the lung. Pflugers Arch 2019;471:519-32. [Crossref] [PubMed]
- Ma T, Fukuda N, Song Y, et al. Lung fluid transport in aquaporin-5 knockout mice. J Clin Invest 2000;105:93-100. [Crossref] [PubMed]
- Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 2008;295:L379-99. [Crossref] [PubMed]
- Woods SJ, Waite AA, O'Dea KP, et al. Kinetic profiling of in vivo lung cellular inflammatory responses to mechanical ventilation. Am J Physiol Lung Cell Mol Physiol 2015;308:L912-21. [Crossref] [PubMed]
- Zhang ZQ, Song YL, Chen ZH, et al. Deletion of aquaporin 5 aggravates acute lung injury induced by Pseudomonas aeruginosa. J Trauma 2011;71:1305-11. [Crossref] [PubMed]
- Liu H, Zhou K, Liao L, et al. Lipoxin A4 receptor agonist BML-111 induces autophagy in alveolar macrophages and protects from acute lung injury by activating MAPK signaling. Respir Res 2018;19:243. [Crossref] [PubMed]
- Tao B, Liu L, Wang N, et al. Effects of hydrogen-rich saline on aquaporin 1, 5 in septic rat lungs. J Surg Res 2016;202:291-8. [Crossref] [PubMed]
- Romano M, Cianci E, Simiele F, et al. Lipoxins and aspirin-triggered lipoxins in resolution of inflammation. Eur J Pharmacol 2015;760:49-63. [Crossref] [PubMed]
- Shi Y, Pan H, Zhang HZ, et al. Lipoxin A4 mitigates experimental autoimmune myocarditis by regulating inflammatory response, NF-kappaB and PI3K/Akt signaling pathway in mice. Eur Rev Med Pharmacol Sci 2017;21:1850-9. [PubMed]
- Park SW, Lee EH, Lee EJ, et al. Apolipoprotein A1 potentiates lipoxin A4 synthesis and recovery of allergen-induced disrupted tight junctions in the airway epithelium. Clin Exp Allergy 2013;43:914-27. [Crossref] [PubMed]


