
A randomised, multicentre open-label phase II study to evaluate the efficacy, tolerability and pharmacokinetics of oral vinorelbine plus cisplatin versus intravenous vinorelbine plus cisplatin in Chinese patients with chemotherapy-naive unresectable or metastatic non-small cell lung cancer
Introduction
Lung cancer is a leading cause of cancer-related mortality worldwide, accounting for approximately a fifth of all cancer related deaths (1). According to a 2012 WHO report, it is the most common form of cancer in men worldwide (1.2 million, 16.7% of the total), with the highest estimated age-standardised incidence rates in central and eastern Europe (53.5 per 100,000) and eastern Asia (50.4 per 100,000) (1). Most cases are diagnosed at locally advanced or metastatic stages. In this setting, chemotherapy has been shown to prolong survival when compared with best supportive care (2). Until 1990, the most active available agents were cisplatin (CDDP)/carboplatin, cyclophosphamide/ifosfamide, mitomycin C, vinblastine/vindesine, and etoposide/teniposide. Since then, new highly active third-generation drugs [vinorelbine (VRL), gemcitabine, and taxanes such as paclitaxel or docetaxel] have been introduced.
Platinum-based doublet regimens, particularly CDDP doublets, are now the mainstay of front-line palliative treatment of advanced non-small cell lung cancer (NSCLC) (3). First-line platinum-based doublet regimens for advanced NSCLC are recommended to be given for four to six cycles, with a maximum of six cycles reserved for patients who respond to therapy and have good tolerance (4,5).
Intravenous VRL, a semi-synthetic vinca alkaloid, has been for many years a reference regimen in combination with CDDP, particularly in Europe. Many attempts were made through large phase III trials conducted around the world to challenge this efficacy; none of them demonstrated better efficacy (6-12). Since the mid-1990s, median survival of patients has been around 10 months.
Currently, intravenous VRL is approved worldwide for treatment of advanced NSCLC and advanced breast cancer. A new oral formulation of VRL has been primarily developed as a line extension of the intravenous formulation. Oral VRL was developed as soft gelatin capsules and is characterised by an absolute bioavailability of 40%, with the same inter-individual variability as the intravenous formulation (13). Its absorption is rapid and bioavailability is not influenced by food (14).
Oral and intravenous doses that achieve equivalent plasma exposure were established at 60 mg/m2 oral for 25 mg/m2 intravenous VRL, and 80 mg/m2 oral for 30 mg/m2 intravenous VRL. In clinical trials, oral VRL has shown efficacy and safety results similar to those of intravenous VRL, both as a single agent and in combination with a platinum salt (15-17).
The metabolism of VRL primarily involves CYP3A4 liver enzymes, except for 4-O-deacetyl-vinorelbine (DVRL), the only active metabolite likely formed by carboxyl-esterases (18). Metabolites are qualitatively similar between oral and intravenous dosing, and bile is the major route of elimination. Urine is only a minor route of elimination (less than 10%) and mostly concerns the parent compound (19). CDDP has a known renal toxicity and is suspected to interact with other drugs at the hepatic level. Therefore, a potential pharmacokinetic drug-drug interaction between VRL and CDDP might arise in the presence of altered hepatic function.
Since 2014, VRL has been approved for treatment of locally advanced, unresectable NSCLC in China (20). Few studies have evaluated the efficacy and tolerability of VRL alongside its pharmacokinetic profile when administered orally or intravenously in Chinese patients. Thus, the present study was designed to evaluate the efficacy (in terms of tumour response) and tolerability of oral and intravenous VRL alone and when administered concomitantly with standard doses of CDDP in Chinese patients with locally advanced/metastatic NSCLC. Pharmacokinetic analyses of oral and intravenous VRL were also performed to provide estimates of individual VRL pharmacokinetic parameters and to investigate the influence of CDDP co-administration on VRL pharmacokinetic in Chinese patients.
Methods
Study design and patients
This was a prospective, multicentre, open-label, randomised phase II trial conducted between January 2008 and September 2009 at six oncology centres in China. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines (ICH-GCP), local laws and applicable regulatory requirements. The study protocol and its related documents were approved by local Ethics Committees (IRB/EC) and Competent Authorities. All patients provided written informed consent for participation in the study.
Eligible patients were men or non-pregnant, non-lactating women, with cytologically or histologically confirmed diagnosis of NSCLC, stage IIIB, IV or in operable relapsed disease at any stage; not previously treated with chemotherapy or immunotherapy, aged 18–75 years (patients above 65 years having no more than 3 co-morbidities affecting cardiac, pulmonary, liver or renal function). Patients had at least one uni-dimensionally measurable lesion according to Response Evaluation Criteria in Solid Tumours (RECIST), version 1.0 (21). Other inclusion criteria were: Karnofsky performance status ≥80%; life-expectancy >3 months; adequate bone marrow, hepatic and renal function, as defined by neutrophils ≥2.0×109/L, platelets ≥100×109/L, haemoglobin >11 g/dL or 6.8 mmol/L, total bilirubin ≤1.5× upper limit of normal (ULN), transaminases <2.5× ULN, alkaline phosphatases <5× ULN, creatinine ≤ULN or creatinine clearance ≥60 mL/min. Patients with a local relapse, which was liable to be treated by radiation therapy, or those who had received radiotherapy within 4 weeks prior to study entry were excluded. Other exclusion criteria were: >10% weight loss within the past 3 months; long-term oxygen therapy; pre-existing symptomatic pleural effusion requiring tapping; active central nervous system disorder, brain metastasis or leptomeningeal involvement; symptomatic neuropathy (sensory) > grade 1 according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC V2.0); cardiac failure or myocardial infarction within past 3 months, uncontrolled hypertension or arrhythmia, unstable diabetes, uncontrolled hypercalcaemia, clinically significant active infection requiring intravenous antibiotics within 2 weeks before study entry; superior vena cava syndrome; malabsorption syndrome or disease significantly affecting gastro-intestinal function or major resection of the stomach or proximal small bowel that could affect absorption of oral VRL; known hypersensitivity to drugs having a chemical structure similar to the study drugs; history of other malignancies, except if more than 5 years without recurrence or patients with a history of adequately treated basal cell carcinoma of the skin or carcinoma in-situ of the cervix.
Treatment methods
Patients were randomly assigned in a 1:1 ratio to receive oral VRL plus CDDP (arm A) or intravenous VRL plus CDDP (arm B). Stratified randomisation was done centrally prior to registration according to a minimisation procedure by centre and disease stage at screening (IIIB, IV or relapse).
Study treatment was initiated within 7 days after randomisation. In the first cycle, VRL was administered orally at 60 mg/m2 on days 1 and 8 in arm A, or intravenously at 25 mg/m2 on days 1 and 8 in arm B in combination with 80 mg/m2 CDDP on day 1 (in both arms). VRL dose was increased to 80 mg/m2 (arm A) or 30 mg/m2 (arm B) in cycles 2–4 in the absence of grade 3–4 neutropenic infection; febrile neutropenia (FN) according to Pizzo’s definition (22); grade 3 neutropenia lasting for more than ≥7 days; or grade 4 neutropenia. One treatment cycle consisted of a 3-week treatment period. Patients were treated for a maximum of 4 cycles, unless there was progressive disease (PD), unacceptable toxicity or the patient refused to continue with the trial.
Oral VRL was supplied as 20, 30, or 40 mg soft gel capsules that had to be taken after a light meal in the presence of a physician or a nurse of the department. The capsules had to be swallowed with a glass of water without chewing or sucking them. Intravenous VRL was administered as a 6–10-minute intravenous infusion under the supervision of a nurse. Oral and intravenous VRL, and commercial CDDP were supplied by Pierre Fabre Médicament to the centres. CDDP was administered intravenously according to the investigational centre’s routine practice either immediately after the intake of oral VRL, or 30 to 60 minutes after completion of intravenous VRL infusion. Anti-emetic treatment with a 5-HT3 antagonist such as ondansetron or granisetron was recommended with oral VRL administration. Preventive anti-emetic treatment was prescribed after CDDP infusion. Hydration on the day of CDDP administration was given according to the investigational centre’s routine practice.
Prior to any dose administration, absolute neutrophil count (ANC) had to be ≥1.5×109/L and platelets ≥75×109/L. If a patient required a cycle delay, both drugs were delayed for a maximum of 2 weeks. Dose modifications were permitted as follows: day 8 of VRL was skipped if grade ≥2 haematological toxicities occurred within a cycle; dose was reduced for subsequent cycles if toxicity grade was ≥3. VRL dose escalation was not permitted for patients who experienced either grade 3 toxicity ≥7 days or one episode of grade 4 haematological toxicity or FN or neutropenic infection during a previous cycle. In case of neurological toxicity of grade ≥2, the treatment was delayed, and the patient reassessed one week later and CDDP dose was reduced by 50% for subsequent cycles. If neurological toxicity grade >2 persisted for >2 weeks or grade ≥3, the patient was discontinued from the study. In case of renal toxicity, CDDP dose was reduced by 50% if creatinine clearance was between 45–54 mL/min despite adequate hydration; if creatinine clearance remained <45 mL/min for more than 2 weeks, the patient was discontinued. Treatment was discontinued for patients with grade 3–4 hearing loss.
Primary prophylactic use of colony stimulating factor was not allowed during the study. Growth factors could be given in case of FN or neutropenic infection according to the centre’s rules. Full supportive care included antibiotics, anti-diarrhoeals, analgesics, and anti-emetics. transfusion of blood products was allowed.
Study procedures
Pre-treatment evaluations included complete medical history, physical examination, audiogram, complete blood cell counts (CBC) and biochemistry tests. During the treatment period, biochemistry tests were performed prior to each treatment cycle; CBC counts were done on days 1 and 8 of each treatment cycle. Tumour assessment was carried out at baseline, then every 2 cycles using the same methods throughout the study to ensure comparability. After the completion of 4 treatment cycles, tumour assessments were performed every 3 months until documented progression. All tumour responses had to be confirmed 4 weeks later. An independent review panel (IRP) was organised in each centre to review all responses and stabilisations. The IRP was kept blinded to the treatment received by the patients. The investigators assessed toxicities during the entire study period by recording adverse events (AE), vital sign measurements and global physical examination.
Study endpoints
The efficacy of two drug combinations (i.e., oral VRL plus CDDP, and intravenous VRL plus CDDP) was determined in terms of tumour response. The primary efficacy endpoint was objective response rate (ORR). Secondary efficacy endpoints included disease control rate (DCR), time to first response, duration of response, time to treatment failure, progression-free survival (PFS) and overall survival (OS).
Statistical methods
The one-sample multiple-testing procedure for phase II clinical trials described by Fleming was used to test the hypothesis that anti-tumour activity would be between a minimum response rate P0 =0.15, for which further investigation was not required, and a response rate PA =0.30 implying efficacy at an acceptable level, with type I error α ≤0.05, and type II error β ≤0.02 (23). Using this hypothesis, the total required sample of evaluable patients was 120 (60 in each treatment arm). All randomised and treated patients were included in the intent-to-treat (ITT) analysis and were analysed for safety. The evaluable population was defined as all eligible patients who underwent a full evaluation of target and non-target lesions and had received at least 2 cycles of study treatment (including patients with PD documented before the second cycle).
The Kaplan-Meier method was used to estimate the duration of response and survival outcomes. Safety analysis was performed on the population evaluable for safety; defined as all patients who received at least one dose of study medication in the assigned treatment arm. All statistical analyses were performed using SAS software, version 8.2 for Windows (SAS Institute, Cary, NC, USA).
Pharmacokinetic assessments
Blood samples for pharmacokinetic evaluation of VRL and its active metabolite 4-O-deacetylvinorelbine (DVRL) were collected during cycle 1 in both treatment arms: on day 1 when VRL was co-administered with CDDP and on day 8 when VRL was administered alone. Pharmacokinetic analysis was conducted using a limited sampling strategy at the following time points for arm A: 0 h (just before oral intake of VRL), 1 h 30 min, 3, 6, 11 and 24 h after oral intake. For arm B, the blood sampling time-points were: 0 h (just before intravenous infusion of VRL), immediately at the end of infusion, 3, 6, 11 and 24 h after infusion.
VRL and its active metabolite, DVRL, were assayed in whole blood by liquid chromatography-tandem mass spectrometry (LC/MS-MS) method with a lower limit of quantification of 0.25 ng/mL (24). Absolute total clearance (Cltot) for the intravenous route and apparent total clearance (Cltot/F) for the oral route were obtained on both days by Bayesian forecasting based on the limited concentrations dataset per individual and using previously developed population pharmacokinetic models (25,26). The drug-drug interaction with CDDP was evaluated separately for each route of administration by comparing individual VRL pharmacokinetic parameters between day 1 (VRL + CDDP) and day 8 (VRL alone). Statistical evaluation was performed by a two-way ANOVA test including “DAY” and “PATIENT” factors (5% nominal risk). For DVRL, no pharmacokinetic parameters could be calculated based on the sparse sampling schedule implemented in the current study. Instead, a descriptive and graphical approach was used to compare individual concentration vs. time profiles between days.
Results
Patient disposition and baseline demographics
Patient disposition is shown in Figure 1. A total of 132 patients were enrolled and randomised (67 in arm A and 65 in arm B). One patient in arm A withdrew consent before undergoing any study treatments; this patient was excluded from analysis. Of 131 randomised and treated patients (ITT population), 74 (56.5%) completed the study treatment as per protocol (i.e., 4 cycles) (38 in arm A and 36 in arm B) and 57 discontinued treatment (28 in arm A and 29 in arm B). The main reason for treatment discontinuation was PD in 42.1% and 30.5% of the patients in arm A and arm B, respectively.

Patient demographics are shown in Table 1. The median (range) age of patients in arm A [52.1 (33.3–71.9) years] was lower than that in arm B [57.9 (25.7–70.9) years]. In both arms, more than half of patients were histologically diagnosed with adenocarcinoma of the lung; all patients with histologically confirmed diagnosis of NSCLC were included in the study. The majority of the patients had metastatic disease (74.2% in arm A and 72.3% in arm B), with 2 or more organs involved at study entry (94.0% in arm A and 96.9% in arm B). All patients were deemed fit to receive the platinum-based combination therapy.
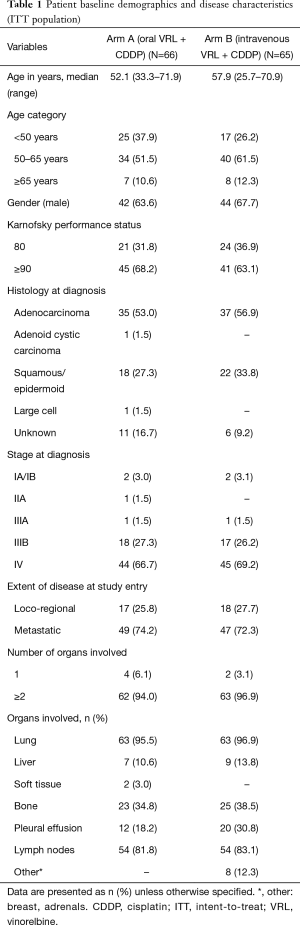
Full table
Drug delivery
The mean duration of therapy was 10.7 weeks in arm A and 11.3 weeks in arm B, with a median of 4 (range, 1–4) cycles. Median relative dose intensity was equivalent between the two arms for VRL (89.3% and 81.4%, respectively) and for CDDP (92.1% and 91.6%, respectively). Ninety-two (71.9%) patients had a VRL dose escalation at cycle 2: 54 patients (84.4%) from 60 to 80 mg/m2 (arm A) and 38 patients (59.4%) from 25 to 30 mg/m2 (arm B). Cycle delay (>2 days) occurred in 25% of the patients in arm A and 31.6% of the patients in arm B. Day 8 was cancelled in 2.3% of the cycles in arm A and in 4.2% of the cycles in arm B. The main reason for day 8 cancellation was haematological toxicity, which occurred in 1 out of 5 cases in arm A and in 7 out of 9 cases in arm B. A total of 10 (15.2%) patients had at least one VRL dose reduction in arm A vs. 17 patients (24.6%) in arm B. CDDP administration was reduced for only 4 (6.1%) patients in arm A and 1 (1.5%) patient in arm B.
Efficacy
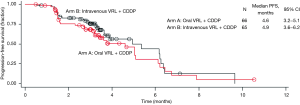
Investigator- and IRP-assessed efficacy results are shown in Table 2. The ORR was calculated based on partial responses achieved by patients in arms A and B. IRP-assessed ORR was 25.8% in arm A and 23.1% in arm B, while investigator-assessed ORR was 19.7% in arm A and 29.2% in arm B. DCR was assessed by both IRP and investigator to be more than 70% in both arms. IRP-assessed time to first response was 1.4 months (95% CI: 1.3–3.0 months) in arm A and 1.5 months (95% CI: 1.3–3.5 months) in arm B, while investigator-assessed time to first response was 1.4 months (95% CI: 1.2–3.0 months) in arm A and 1.4 months (95% CI: 1.2–2.8 months) in arm B. The median PFS was 4.6 months (95% CI: 3.2–5.1 months) in arm A and 4.9 months (95% CI: 3.6–6.2 months) in arm B. With a censor rate of 30% in arm A and 23% in arm B, the median OS was 16.1 months (95% CI: 10.0–24.9 months) in arm A and 19.0 months (95% CI: 11.9–25.3 months) in arm B (Figure 2).
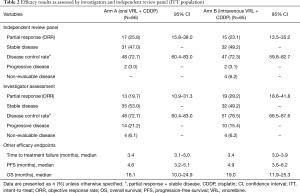
Full table
Safety and tolerability
Haematological toxicity was common in both treatment arms with grade 3/4 neutropenia occurring in 29 (43.9%) patients in arm A and 56 (86.2%) patients in arm B (Table 3). Grade 3/4 haematological toxicity was less commonly reported in patients receiving oral VRL than intravenous VRL. The incidence of FN was low; only 4 (6.1%) patients in arm A and 6 (9.2%) patients in arm B reported FN. Five patients required red blood cell transfusion: one patient in arm A and four patients in arm B.
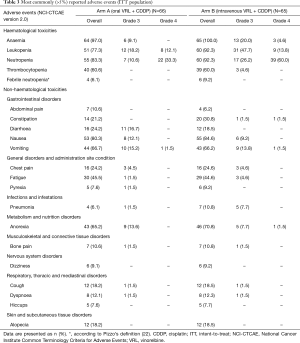
Full table
The most frequent non-haematological AE were nausea, vomiting and anorexia, but the incidence of grade 3/4 events was low (Table 3). Diarrhoea was reported in 16 (24.2%) and 12 (18.5%) patients in arm A and arm B, respectively. There were three deaths during the study. One patient (arm A) died due to PD and two patients (one in each arm) died from pneumonia during cycle one; both events were assessed as possibly related to study treatment.
Pharmacokinetics analyses
Pharmacokinetic analyses were performed on blood samples taken from 21 patients who received oral VRL (arm A) and 17 patients who received intravenous VRL (arm B) on days 1 and 8.
VRL and DVRL plasma concentration profiles
Individual plasma concentration profiles of VRL and its metabolite, DVRL, were compared between day 1 and day 8 (Figure 3). For both routes of administration, individual VRL plasma concentration profiles overlapped when day 1 data were superimposed upon day 8 data. There was little to no accumulation of VRL from day 1 to day 8 (Figures 3A,B). DVRL accumulated at low concentration levels (below 10 ng/mL) after oral administration (Figure 3C) or intravenous infusion (Figure 3D).
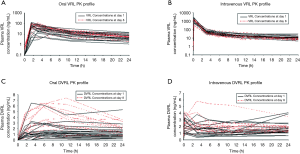
Clearance of oral and intravenous VRL
The mean apparent clearance of oral VRL was similar between the two treatment modalities: 175±93.6 L/h (VRL + CDDP) vs. 138±56.5 L/h (VRL alone). As depicted in Figure 4A, no obvious trend (decrease or increase between day 1 and day 8) was observed, indicating the absence of CDDP effect on oral VRL pharmacokinetics. The mean total clearance of intravenous VRL appeared tended to be lower (by ~25%) when combined with CDDP (26.1±10.5 L/h) than when given alone (34.6±8.82 L/h; P<0.05). However, this difference was observed in only a few patients. As depicted in Figure 4B, an increase in total clearance from day 1 to day 8 was obvious only for patients who exhibited low clearance (below 20 L/h) on day 1 before returning to values between 20–40 L/h on day 8. Consequently, the mean total body clearance on day 1 was lower than on day 8 for this subset of patients.

Discussion
In the present study, we evaluated the efficacy of two drug combinations in Chinese patients with NSCLC in terms of tumour response. The primary efficacy endpoint of this study, ORR, was similar for oral and intravenous routes of VRL in combination with CDDP, thus demonstrating the efficacy of both routes in first-line treatment of advanced NSCLC in Chinese patients. Other secondary efficacy parameters, including DCR, median PFS, and median OS, support this conclusion. These results are also consistent with previous experience with oral and intravenous VRL/CDDP combination regimens in European patients, which were associated with similar response rates (6,11,13,27).
Discrepancies between IRP-assessed and investigator-assessed efficacy results were observed and were expected. Independent reviews are conducted primarily to discern and minimise bias that may be introduced by the investigators (28). As such, blinded independent reviews are recommended for clinical trials studying tumour response or disease progression (28-32).
The two regimens had similar safety profiles, in line with the previously reported randomised studies with this combination (11,15,27). Neutropenia, the most commonly reported haematological toxicity, was rarely complicated with FN, which is particularly encouraging in light of high rates of FN reported in studies with other agents. First-line agents such as paclitaxel and docetaxel have been associated with high incidence of bone marrow suppression and FN (up to 26% of patients), including high incidence rates in the first cycle (33,34). This has been a cause of additional patient care and treatment costs. Concerning non-haematological toxicity, gastro-intestinal side effects were frequent but could be easily managed by anti-emetics and dietary education.
Oral chemotherapy, when effective, may offer better convenience and advantages for patients and physicians over standard intravenous chemotherapy (35). Current research is focused on developing oral formulations active against NSCLC, and several agents are already approved or are in development (36,37). The availability of effective oral chemotherapy may offer more flexibility to patients who are living in remote areas or are far from oncology clinics (38). Oral chemotherapy also reduces anxiety in patients who are afraid of injections (37,39), and may be more appropriate when venous access is problematic. Our results demonstrated better tolerability with oral VRL than with intravenous VRL; less patients receiving oral VRL than intravenous VRL discontinued day 8 treatment due to toxicity. Indeed, studies have shown that most patients prefer oral to intravenous therapy, assuming similar efficacy (37,40). Patient preference for oral vs. intravenous VRL administration was evaluated in a randomised trial in advanced NSCLC. Oral VRL plus platinum salt was preferred by 3 out of 4 patients; moreover, patients reported that their everyday life was less affected due to less time spent at the clinic and the possibility of taking the day 8 dose at home (41).
In addition to assessing efficacy, another objective of this study was to assess the pharmacokinetics parameters of oral and intravenous VRL in Chinese patients and examine potential drug-drug interaction with CDDP. The risk of VRL interaction with CDDP could be considered low, since VRL is only poorly eliminated by the kidney. Thus far, amongst numerous CDDP-VRL combination studies, only two trials have explored potential pharmacokinetic drug-drug interactions of orally or intravenously administered VRL (42,43). Both studies were conducted in European populations and did not demonstrate any CDDP-VRL interaction.
Our study presented valuable insights into the pharmacokinetic profile of oral/intravenous VRL, alongside efficacy and tolerability, in an all-Chinese patient population. We observed no effect of CDDP on the pharmacokinetic behaviour of VRL. With oral administration of VRL, apparent clearance was not affected by CDDP co-administration. With intravenous administration, VRL clearance was affected by CDDP, but only in a small subset of patients. For the majority of patients, there appeared to be no effect of CDDP. Similarly, although few patients exhibited higher DVRL concentrations, there were no observed difference in clinical responses or incidence of AEs compared to the other patients. The present study in Chinese patients thus confirmed the previous observations regarding lack of interaction between VRL (oral or intravenous) and CDDP in European populations (42,43).
Conclusions
In summary, oral VRL in combination with CDDP is effective and well-tolerated in Chinese patients with advanced NSCLC. The efficacy of oral VRL was comparable to that of intravenous VRL, as suggested by ORR, DCR, PFS and OS results. Furthermore, the safety profile of both routes was also similar. Similar pharmacokinetic behaviour was observed for oral and intravenous VRL, independent of CDDP co-administration. Oral VRL is an attractive option for first-line treatment of NSCLC, combining treatment convenience with a high level of efficacy and safety.
Acknowledgments
Funding: This study was funded by Pierre Fabre Médicament, France. Y Yang was supported by Chinese National Natural Science Foundation project (Grant No.81602011), Outstanding Young Talents Program of Sun Yat-sen University Cancer Center (16zxyc03) and Central Basic Scientific Research Fund for Colleges-Young Teacher Training Program of Sun Yatsen University (17ykpy85). L Zhang has received research support from the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDA12020101 to J.D.).
Footnote
Conflicts of Interest: JP Burillon, M Riggi, A Petain, P Ferre are employees of Institut de Recherche Pierre Fabre, France (the sponsor of the study). The other authors have no conflicts of interest to declare.
Ethics Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol and its related documents were approved by local Ethics Committees (IRB/EC) and Competent Authorities, namely, the ethics committees of Sun Yat-sen University Cancer Center, Beijing Cancer Hospital, Fujian Provincial Tumor Hospital, Fudan University Cancer Hospital, and Zhejiang Cancer Hospital. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
References
- World Health Organization. Globocan 2012. Lung cancer. Estimated Incidence, Mortality and Prevalence Worldwide in 2012. 2012. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx/. Accessed 15 June 2015.
- Clinical practice guidelines for the treatment of unresectable non-small-cell lung cancer. Adopted on May 16, 1997 by the American Society of Clinical Oncology. J Clin Oncol 1997;15:2996-3018. [Crossref] [PubMed]
- Pfister DG, Johnson DH, Azzoli CG, et al. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol 2004;22:330-53. [Crossref] [PubMed]
- Azzoli CG, Baker S Jr, Temin S, et al. American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol 2009;27:6251-66. [Crossref] [PubMed]
- Coate LE, Shepherd FA. Maintenance therapy in advanced non-small cell lung cancer: evolution, tolerability and outcomes. Ther Adv Med Oncol 2011;3:139-57. [Crossref] [PubMed]
- Douillard JY, Gervais R, Dabouis G, et al. Sequential two-line strategy for stage IV non-small-cell lung cancer: docetaxel-cisplatin versus vinorelbine-cisplatin followed by cross-over to single-agent docetaxel or vinorelbine at progression: final results of a randomised phase II study. Ann Oncol 2005;16:81-9. [Crossref] [PubMed]
- Fossella F, Pereira JR, von Pawel J, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. J Clin Oncol 2003;21:3016-24. [Crossref] [PubMed]
- Gebbia V, Galetta D, Caruso M, et al. Gemcitabine and cisplatin versus vinorelbine and cisplatin versus ifosfamide+gemcitabine followed by vinorelbine and cisplatin versus vinorelbine and cisplatin followed by ifosfamide and gemcitabine in stage IIIB-IV non small cell lung carcinoma: a prospective randomized phase III trial of the Gruppo Oncologico Italia Meridionale. Lung Cancer 2003;39:179-89. [Crossref] [PubMed]
- Georgoulias V, Ardavanis A, Tsiafaki X, et al. Vinorelbine plus cisplatin versus docetaxel plus gemcitabine in advanced non-small-cell lung cancer: a phase III randomized trial. J Clin Oncol 2005;23:2937-45. [Crossref] [PubMed]
- Kelly K, Crowley J, Bunn PA Jr, et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non--small-cell lung cancer: a Southwest Oncology Group trial. J Clin Oncol 2001;19:3210-8. [Crossref] [PubMed]
- Martoni A, Marino A, Sperandi F, et al. Multicentre randomised phase III study comparing the same dose and schedule of cisplatin plus the same schedule of vinorelbine or gemcitabine in advanced non-small cell lung cancer. Eur J Cancer 2005;41:81-92. [Crossref] [PubMed]
- Scagliotti GV, De Marinis F, Rinaldi M, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol 2002;20:4285-91. [Crossref] [PubMed]
- Marty M, Fumoleau P, Adenis A, et al. Oral vinorelbine pharmacokinetics and absolute bioavailability study in patients with solid tumors. Ann Oncol 2001;12:1643-9. [Crossref] [PubMed]
- Bugat R, Variol P, Roche H, et al. The effects of food on the pharmacokinetic profile of oral vinorelbine. Cancer Chemother Pharmacol 2002;50:285-90. [Crossref] [PubMed]
- Jassem J, Kosmidis P, Ramlau R, et al. Oral vinorelbine in combination with cisplatin: a novel active regimen in advanced non-small-cell lung cancer. Ann Oncol 2003;14:1634-9. [Crossref] [PubMed]
- Jassem J, Ramlau R, Karnicka-Mlodkowska H, et al. A multicenter randomized phase II study of oral vs. intravenous vinorelbine in advanced non-small-cell lung cancer patients. Ann Oncol 2001;12:1375-81. [Crossref] [PubMed]
- Lena MD, Ramlau R, Hansen O, et al. Phase II trial of oral vinorelbine in combination with cisplatin followed by consolidation therapy with oral vinorelbine in advanced NSCLC. Lung Cancer 2005;48:129-35. [Crossref] [PubMed]
- Beulz-Riché D, Grude P, Puozzo C, et al. Characterization of human cytochrome P450 isoenzymes involved in the metabolism of vinorelbine. Fundam Clin Pharmacol 2005;19:545-53. [Crossref] [PubMed]
- Focan C, Kreutz F, Leroy I, et al. Pharmacokinetics and mass-balance elimination of 3H-vinorelbine following i.v. and oral administration to patients. Proceedings of 92nd Annual Meeting of the American Association for Cancer Research, 2001:abstr 2064.
- Médicament PF. Pierre Fabre Médicament obtains MA in China for NAVELBINE®Oral (Vinorelbine*). 2014. Available online: https://www.pierre-fabre.com/en/news/pierre-fabre-medicament-obtains-ma-in-china-for-navelbineroral-vinorelbine. Accessed 31 May 2019.
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [Crossref] [PubMed]
- Pizzo PA. Management of fever in patients with cancer and treatment-induced neutropenia. N Engl J Med 1993;328:1323-32. [Crossref] [PubMed]
- Fleming TR. One-sample multiple testing procedure for phase II clinical trials. Biometrics 1982;38:143-51. [Crossref] [PubMed]
- Van Heugen JC, De Graeve J, Zorza G, et al. New sensitive liquid chromatography method coupled with tandem mass spectrometric detection for the clinical analysis of vinorelbine and its metabolites in blood, plasma, urine and faeces. J Chromatogr A 2001;926:11-20. [Crossref] [PubMed]
- Nguyen L, Tranchand B, Puozzo C, et al. Population pharmacokinetics model and limited sampling strategy for intravenous vinorelbine derived from phase I clinical trials. Br J Clin Pharmacol 2002;53:459-68. [Crossref] [PubMed]
- Variol P, Nguyen L, Tranchand B, et al. A simultaneous oral/intravenous population pharmacokinetic model for vinorelbine. Eur J Clin Pharmacol 2002;58:467-76. [Crossref] [PubMed]
- Gebbia V, Galetta D, Lorusso V, et al. Cisplatin plus weekly vinorelbine versus cisplatin plus vinorelbine on days 1 and 8 in advanced non-small cell lung cancer: a prospective randomized phase III trial of the G.O.I.M. (Gruppo Oncologico Italia Meridionale). Lung Cancer 2008;61:369-77. [Crossref] [PubMed]
- Zhang J, Zhang Y, Tang S, et al. Systematic bias between blinded independent central review and local assessment: literature review and analyses of 76 phase III randomised controlled trials in 45 688 patients with advanced solid tumour. BMJ Open 2018;8:e017240. [Crossref] [PubMed]
- Amit O, Bushnell W, Dodd L, et al. Blinded independent central review of the progression-free survival endpoint. Oncologist 2010;15:492-5. [Crossref] [PubMed]
- Floquet A, Vergote I, Colombo N, et al. Progression-free survival by local investigator versus independent central review: comparative analysis of the AGO-OVAR16 Trial. Gynecol Oncol 2015;136:37-42. [Crossref] [PubMed]
- Ford RR, Ford RW, O'Neal M, et al. Investigator and independent review committee exploratory assessment and verification of tumor response in a non-Hodgkin lymphoma study. Leuk Lymphoma 2017;58:1332-40. [Crossref] [PubMed]
- Tang PA, Pond GR, Chen EX. Influence of an independent review committee on assessment of response rate and progression-free survival in phase III clinical trials. Ann Oncol 2010;21:19-26. [Crossref] [PubMed]
- Millward MJ, Boyer MJ, Lehnert M, et al. Docetaxel and carboplatin is an active regimen in advanced non-small-cell lung cancer: a phase II study in Caucasian and Asian patients. Ann Oncol 2003;14:449-54. [Crossref] [PubMed]
- Weycker D, Li X, Edelsberg J, et al. Risk and Consequences of Chemotherapy-Induced Febrile Neutropenia in Patients With Metastatic Solid Tumors. J Oncol Pract 2015;11:47-54. [Crossref] [PubMed]
- Jensen LH, Osterlind K, Rytter C. Randomized cross-over study of patient preference for oral or intravenous vinorelbine in combination with carboplatin in the treatment of advanced NSCLC. Lung Cancer 2008;62:85-91. [Crossref] [PubMed]
- Partridge AH, Avorn J, Wang PS, et al. Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst 2002;94:652-61. [Crossref] [PubMed]
- Sharma S. Patient selection for oral chemotherapy. Oncology (Williston Park) 2001;15:33-5. [PubMed]
- Damle B, Ravandi F, Kaul S, et al. Effect of food on the oral bioavailability of UFT and leucovorin in cancer patients. Clin Cancer Res 2001;7:517-23. [PubMed]
- Faithfull S, Deery P. Implementation of capecitabine (Xeloda) into a cancer centre: UK experience. Eur J Oncol Nurs 2004;8 Suppl 1:S54-62. [Crossref] [PubMed]
- Chau I, Legge S, Fumoleau P. The vital role of education and information in patients receiving capecitabine (Xeloda). Eur J Oncol Nurs 2004;8 Suppl 1:S41-53. [Crossref] [PubMed]
- Georgoulias V. Docetaxel (taxotere) in the treatment of non-small cell lung cancer. Curr Med Chem 2002;9:869-77. [Crossref] [PubMed]
- Delord JP, Puozzo C, Lefresne F, et al. Combination chemotherapy of vinorelbine and cisplatin: a phase I pharmacokinetic study in patients with metastatic solid tumors. Anticancer Res 2009;29:553-60. [PubMed]
- Levêque D, Jehl F, Quoix E, et al. Clinical pharmacokinetics of vinorelbine alone and combined with cisplatin. J Clin Pharmacol 1992;32:1096-8. [PubMed]


