
Endobronchial ultrasound-guided intranodal forceps biopsy (EBUS-IFB)—technical review
Introduction
With the introduction of endobronchial ultrasound (EBUS)-guided transbronchial needle aspiration (EBUS-TBNA) in 2003, bronchoscopists are able to perform guided sampling of structures next to the airways (1). In the following decade, EBUS-TBNA has become the standard of care for lung cancer staging, recommended by American College of Chest Physicians (ACCP) as the procedure of choice over mediastinoscopy (2,3). As the technique for EBUS-TBNA becomes standardized, there are new challenges that need to be addressed, specifically in the areas of tissue acquisition for molecular testing and for the diagnosis of certain conditions, such as lymphoma and sarcoidosis.
While initial studies using EBUS-TBNA samples to complete molecular analysis demonstrated success rates ranging from 83–97%, the increasing demand for tumor tissue poses a challenge to specimen handling (4-8). Current guidelines suggest that in the setting of mediastinal staging, EBUS-TBNA provides adequate material for diagnostic purposes with 3 needle passes (3). When molecular profiling is needed, additional needle passes may be required, with the optimal number of specimens often based on operator judgement and the type of molecular analysis being performed. Furthermore, PD-L1 testing on EBUS-TBNA samples has not been validated with any of the commercially available platforms (9). Moreover, there is evidence to suggest that EBUS-TBNA samples PD-L1 status are discordant to that of the surgical resected specimen of the primary site of lung cancer (10).
The diagnostic yield of EBUS-TBNA for sarcoidosis is lower than that for carcinoma (3,11,12). While the diagnosis of sarcoidosis can be enhanced with transbronchial and endobronchial lung biopsy, these additional steps may increase the risk of bleeding and pneumothorax (13-15). While the diagnostic yield of EBUS-TBNA for sarcoidosis has been reported at greater than 90% in some studies, this result may not be representative of practices in which experienced cytopathology services are not available.
The diagnostic yield of EBUS-TBNA for lymphoma has been reported to be lower than that for either carcinoma or benign conditions such as sarcoidosis. While EBUS-TBNA may be adequate for diagnosing relapsed lymphoma, it has not performed well with de novo lymphoma (16-18). Non-Hodgkin’s and Hodgkin’s lymphoma treatment depend on specific subtyping and histologic grade. Therefore, definitive subtyping is essential for management and requires the evaluation of cell morphology, immunophenotype, and tissue architecture.
EBUS-guided intranodal forceps biopsies (EBUS-IFB) is a technique in which small miniforceps are passed into targeted lymph nodes following EBUS-TBNA needle puncture. The material obtained via EBUS-IFB can be processed as a histological specimen and has been shown to improve the overall diagnostic yield of EBUS procedures when combined with TBNA (19-24). All authors of this paper routinely perform EBUS-IFB in their respective institutions. Here, we describe the technique for EBUS-IFB in patients with mediastinal and hilar lymphadenopathy and discuss indications, contraindications, technique, and available literature for the procedure.
Intranodal forceps biopsy (IFB)
Nomenclature
IFB has been described in the literature as micro- or mini-forceps biopsy (MFB), transbronchial forceps biopsy (TBFB), and transbronchial needle forceps (TBFN) (19-24). In this manuscript, we use the term IFB to describe the procedure, and propose that this more accurately reflects the procedural elements of obtaining forceps biopsy specimens from within a targeted lymph node.
Equipment
EBUS-IFB is performed utilizing a standard EBUS bronchoscope, TBNA needle and 1.0-mm miniforceps (Figure 1). While 22-gauge needles have been used for TBNA in most EBUS-IFB procedures in the literature, others have reported success using 19-, 21- and even 25-gauge EBUS-TBNA needles. White light bronchoscopy is performed initially using a conventional bronchoscope for airway inspection as per routine clinical practice.
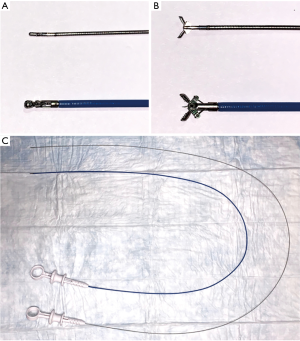
Comparison of miniforceps and regular pulmonary forceps. (A) Miniforceps and regular spiked pulmonary forceps in closed position; (B) miniforceps and regular spiked pulmonary forceps in open position; (C) comparison of the length of miniforceps verses regular spiked pulmonary forceps.
Technique
Following EBUS-TBNA, the airway mucosa is punctured 4 to 5 times under ultrasound guidance using the EBUS-TBNA needle. This creates a defect in the airway mucosa as well as a tract for introduction of miniforceps into the targeted lymph node. With the EBUS bronchoscope in steady position, the aspiration needle is withdrawn from the working channel and the miniforceps advanced into the target under EBUS guidance. Direct endoscopic visualization of the mucosal puncture site is often not possible and therefore may not be a reliable landmark for miniforceps insertion. To facilitate miniforceps insertion into the lymph node, the operator must maintain a consistent EBUS image of the lymph node to avoid any rotational or cranial/caudal deviation of the EBUS bronchoscope that may result in misalignment of the miniforceps to the mucosal puncture site (Figure 2). In some cases, a needle tract will be created during TBNA puncture which may be used as a reference to guide miniforceps insertion; alternatively, intranodal landmarks such as hyper or hypoechoic spots may also be used as landmarks to assist with guiding the miniforceps into the lymph node. It may be advisable to begin miniforceps insertion slightly proximal to the target tract as the miniforceps may slide along the mucosa prior to entering the mucosal defect of the target tract. If a 19-gauge needle is used, the needle entry alone should create a tract of sufficient size for advancing the miniforceps into the target. Once inside the target lesion, the miniforceps are opened and biopsy specimens are taken under continuous EBUS surveillance (Figure 3).

EBUS-IFB procedure (25). EBUS, endobronchial ultrasound; IFB, intranodal forceps biopsy. Available online: http://www.asvide.com/watch/32949
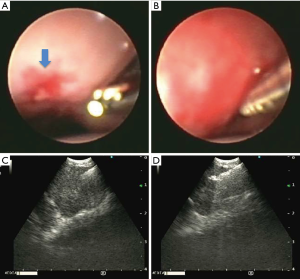
Endobronchial view and ultrasound view. (A) Endobronchial view with microforceps extended out of the working channel. Blue arrow is indicating the mucosal defect made by TBNA; (B) miniforceps inserted into the bronchial airway wall; (C) endobronchial ultrasound view of the miniforceps in the lymph node; (D) endobronchial ultrasound view of the opening and advancing of miniforceps in the lymph node. TBNA, transbronchial needle aspiration.
Specimen handling
Specimens obtained using EBUS-TBNA are processed per institutional protocol for cytological specimens. EBUS-IFB biopsies should be processed as histology specimens (Figure 4). Samples are placed into formalin solution for permanent fixation or into saline for culture. Frozen section can be performed if intraprocedural feedback is desired and rapid on-site evaluation of EBUS-TBNA specimens is not available (Figure 5). Touch preparations can be done with IFB specimens for immediate on-site evaluation, though this practice is highly variable amongst institutions. Due to the relatively small size of IFB specimens, efforts should be placed on tissue conservation. It is advisable to solicit input from institutional cytologists and pathologists regarding the preferred manner in which specimens are processed.

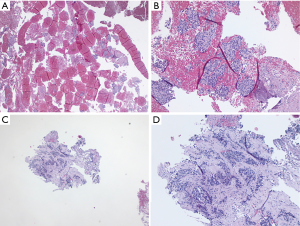
Cytology of EBUS-TBNA and histology of EBUS-IFB. (A) Low magnification (H&E stain, 10×) of EBUS-TBNA 25G aspiration; (B) high magnification (H&E stain, 40×) of EBUS-TBNA 25G aspiration; (C) low magnification (H&E stain, 10×) of EBUS-IFB; (D) high magnification (H&E stain, 40×) of EBUS-IFB. Diagnosis was a renal cell carcinoma. EBUS, endobronchial ultrasound; TBNA, transbronchial needle aspiration; IFB, intranodal forceps biopsy; EBUS-TBNA, EBUS-guided TBNA; EBUS-IFB, EBUS-guided IFB.
Histology of EBUS-TBNA and EBUS-IFB. (A) Low magnification (H&E stain, 10×) of EBUS-TBNA 19G aspiration; (B) high magnification (H&E stain, 40×) of EBUS-TBNA 19G aspiration; (C) low magnification (H&E stain, 10×) of EBUS-IFB; (D) high magnification (H&E stain, 40×) of EBUS-IFB. Diagnosis was squamous cell lung cancer. EBUS, endobronchial ultrasound; TBNA, transbronchial needle aspiration; IFB, intranodal forceps biopsy; EBUS-TBNA, EBUS-guided TBNA; EBUS-IFB, EBUS-guided IFB.
Indications
EBUS-IFB is complementary to traditional EBUS-TBNA, and is performed following specimen collection with EBUS-TBNA. EBUS-IFB may be performed in any clinical scenario in which additional tissue is requested for purposes such as molecular marker analysis or to assist with making a diagnosis of centrally located thoracic processes that are amenable to EBUS-TBNA. EBUS-IFB provides histology specimens and therefore may be used in cases where “core” biopsy specimens are desired. All mediastinal and hilar lymph node stations accessible via EBUS-TBNA are accessible using EBUS-IFB.
Contraindications
Contraindications to EBUS-IFB are similar to that of EBUS-TBNA, generally related to patient’s fitness to undergo bronchoscopy as well as to tolerate moderate sedation or general anesthesia. Procedures should be avoided in patients at high risk for pulmonary and cardiovascular decompensation (i.e., severe refractory hypoxemia, hypotension, and arrhythmias), bleeding (i.e., systemic anticoagulation and thrombocytopenia), in those who are unable to give informed consent, and in those who have had adverse reactions to anesthesia. As with EBUS-TBNA, systemic anticoagulation should be discontinued prior to procedures if possible.
Safety
As a complementary procedure to EBUS-TBNA, EBUS-IFB does not significantly affect the safety profile or workflow of bronchoscopy procedures. Of approximately 300 EBUS-IFB procedures in published literature, the overall complication rate is 1.5%, which is comparable to the complication rate associated with EBUS-TBNA. Three patients experienced bleeding, controlled locally without the need for transfusion and no deaths have been associated with the procedure. Management of bleeding from EBUS-IFB is no different than that with EBUS-TBNA. Often, observation alone allows enough time for bleeding to stop. If needed, EBUS scope balloon can be used to occlude the entry point in the airway wall and provide temporary balloon tamponade to stop bleeding. Antimicrobial prophylaxis has not been thoroughly evaluated and practice varies across centers. The addition of EBUS-IFB does not significantly prolong procedure time, adding on average less than 4 minutes to EBUS-TBNA procedures in one publication (21). While pneumomediastinum has been reported with EBUS-TBNA, we do not recommend routine use of CT scans in assessment, as most are self-limiting and will resolve without intervention.
Clinical evidence
IFB was first performed without EBUS guidance in an effort to increase diagnostic yield when paired with conventional TBNA (19). Oki and colleagues performed procedures on 22 consecutive patients with enlarged subcarinal lymph nodes using a 19-gauge TBNA needle and 1.15-mm miniforceps without ultrasound guidance. Diagnostic tissue was obtained by IFB in 3 patients in which TBNA was non-diagnostic. There was a single case of pneumomediastinum (19). In 2008, Herth et al. reported on the safety and efficacy of obtaining histologic specimens from subcarinal lesions larger than 2.5 cm using EBUS-TBNA and EBUS-IFB (20). Seventy-five patients underwent EBUS-TBNA with 22-gauge needle, followed by needle puncture using a 19-gauge needle through a conventional bronchoscope. IFB was subsequently performed through the defect made by the 19-gauge needle using EBUS guidance, and all procedures were performed under general anesthesia through a rigid bronchoscope. A specific diagnosis was made in 36% of patients using the 22-gauge needle, 49% with the 19-gauge needle, and 88% with the IFB. The increase in diagnostic yield with IFB was most pronounced in patients with sarcoidosis (88% vs. 36%, P=0.001) and lymphoma (81% vs. 35%, P=0.038), and no complications were noted (20). In 2011, Chrissian and colleagues prospectively evaluated EBUS-TBNA and EBUS-IFB in 74 lymph node stations in 50 patients. The overall diagnostic yield was 81% and 91%, for EBUS-TBNA and EBUS-IFB respectively (21). The overall diagnostic yield combining both modalities was 97%, which was a statistically significant improvement compared with performing EBUS-TBNA alone (P<0.001). There were no complications and the addition of EBUS-IFB to EBUS-TBNA did not significantly lengthen procedure time (21). A recent study examined 91 patients who underwent both EBUS-TBNA and EBUS-IFB noted that EBUS-IFB lead to additional pathologic diagnosis in 16.2% of non-diagnostic EBUS-TBNA samples, of which 66% were non-caseating granulomas (26). Interestingly, Bramley et al. reported the combined use of electrocautery knife and 1.9-mm spiked forceps to obtain lymph node tissue to be safe and more effective in detection of granulomatous disease and provided larger specimens for clinical studies. However, it is worth of noting that EBUS-TBNA had higher sensitivity for detecting malignancy than EBUS-cautery assisted TBFB (ca-TBFB) (27). Thus, these data overall support the idea that EBUS-TBNA and EBUS-IFB are complementary techniques in patient management. The relevant studies pertaining to EBUS-IFB are summarized in Table 1.
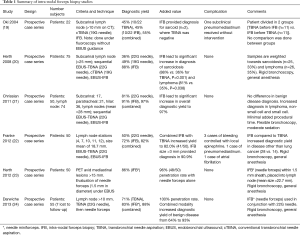
Full table
Summary of intra-nodal forceps biopsy studies
*, needle miniforceps. IFB, intra-nodal forceps biopsy; TBNA, transbronchial needle aspiration; EBUS, endobronchial ultrasound; cTBNA, conventional transbronchial needle aspiration.
Discussion
EBUS-TBNA has greatly altered the manner in which lung cancer is diagnosed and staged. With advances in cancer treatment, bronchoscopists are asked to provide increasing amount of material for molecular testing in a safe and minimally invasive way. As a complementary technique to EBUS-TBNA, EBUS-IFB combines the EBUS guidance with miniforceps biopsy to obtain histology specimen for additional tissue acquisition. However, in clinical practice, EBUS-IFB is underutilized. Several factors contribute to the current state. One, lack of familiarity with EBUS-IFB technique prohibit its use when EBUS-TBNA fails to provide adequate samples. Two, with less clinical use, physicians often fail to think of the indications that EBUS-IFB should be considered. Three, unfamiliarity with the equipment and the added cost of the miniforceps are prohibitive for adoption in resource poor regions. EBUS-TBNA has been widely adopted and most bronchoscopists are familiar with the technique. As a complementary procedure, EBUS-IFB may be well suited for several clinical scenarios.
First, EBUS-IFB may be useful for cases in which additional material is required for advanced molecular analysis such as EGFR, ALK, PD-L1 testing or next generation sequence testing. EBUS-IFB biopsies is submitted as core specimens for processing. While some institutional practices may be capable of performing these assays using cytological specimens obtained by EBUS-TBNA, this practice appears to be heterogeneous across centers at the present time. Submitting core specimens in addition to cytology specimens may improve the likelihood for specimen adequacy for such tests, thereby obviating the need for additional more invasive procedures, such as mediastinoscopy or thoracoscopy. Of note, there is a 19-gauge EBUS needle for core sample acquisition. In a recent study comparing 22- to 19-gauge needle for tissue acquisition, the authors found similar diagnostic yield, but 19-gauge had more tissue by weight and more tumor cells per sample (28). However, in a separate study, Chaddha et al. noted that 19-gauge needle did not provide an increase in diagnostic yield and the samples are often more bloody (29). From our experience, blood contamination is not an issue in EBUS-IFB. Of note, there is no comparison of tissue acquisition between 19-gauge needle and IFB, which remains an active area for clinical research. Additional randomized controlled prospective studies will need to be performed to ascertain the added value of the EBUS-IFB in the era of molecular testing.
For diagnostic procedures such as in cases of suspected lymphoma or sarcoidosis, combining EBUS-TBNA with EBUS-IFB may improve the overall diagnostic yield of bronchoscopy, without significantly increasing procedure time or complications. While the difference in diagnostic yield between EBUS-TBNA and EBUS-IFB was not statistically significant (81% vs. 91%, respectively), the combined yield of 97% for both procedures represented a statistically significant improvement over EBUS-TBNA alone, suggesting that EBUS-IFB may provide a diagnosis in cases where EBUS-TBNA does not (21). This study supports the notion that the benefit of EBUS-IFB is greatest when used as a complementary procedure to EBUS-TBNA, not to be used as a replacement for EBUS-TBNA. Additionally, the ability to provide a histology specimen may enable pathologists to more readily identify diagnostic elements in the lymph node (20). This may be advantageous in centers with less experience using EBUS-TBNA as learning curves for both bronchoscopists and cytopathologists have been shown to influence the diagnostic yield of procedures (30). Whether EBUS-IFB is a superior technique to EBUS-TNBA remains to be determined by future studies.
The influence of EBUS-IFB for lymphoma remains unclear, due largely to the relatively small sample sizes reported in available literature. Herth et al. reported in 75 patients an overall improvement in diagnostic yield using EBUS-IFB, which was most pronounced in lymphoma cases (81% vs. 35%, P=0.038) (20). In a separate study, EBUS-IFB provided diagnosis of lymphoma in 4 of 4 (100%) lymph nodes compared to 0 of 4 lymph nodes using EBUS-TBNA (1 Hodgkin’s lymphoma and 3 non-Hodgkin’s lymphoma) despite the use of flow cytometry in all cases. All miniforceps samples were adequate to guide management and all cases of lymphoma were new diagnoses (21). In a recent study, EBUS-TBNA was able to diagnose and subtype lymphoma in 67% (95% CI, 0.45–0.88) of patients with de novo lymphoma and 81% (95% CI, 0.70–0.91) with relapsed lymphoma (31). Taken these studies together, in cases of suspected lymphoma, EBUS-IFB should be considered in addition to EBUS-TBNA (Figure 6A,B).
EBUS-IFB was initially proposed as a method to improve the overall diagnostic yield of bronchoscopy procedures. While the yield of EBUS-TBNA remains excellent for the diagnosis of carcinoma, challenges remain with increased demand for additional tissue for advanced genetic testing as well as the need to improve diagnostic yields for certain conditions such as lymphoma, sarcoid, and infection (Figure 6C,D). The bronchoscopists are faced with question of how to accomplish additional tissue acquisition more effectively, efficiently and safely using a minimally invasive technique. We propose that EBUS-IFB be considered for such instances and suggest that, when combined with EBUS-TBNA, this technique may improve the overall utility of bronchoscopy to provide diagnostic, staging and specimen acquisition.

Acknowledgments
None.
Footnote
Conflicts of Interest: G Cheng, A Mahajan, S Oh, S Benzaquen and A Chen served as consultants for Boston Scientific.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Yasufuku K, Chiyo M, Sekine Y, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest 2004;126:122-8. [Crossref] [PubMed]
- Detterbeck FC, Lewis SZ, Diekemper R, et al. Executive Summary: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:7S-37S.
- Wahidi MM, Herth F, Yasufuku K, et al. Technical Aspects of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: CHEST Guideline and Expert Panel Report. Chest 2016;149:816-35. [Crossref] [PubMed]
- Hurter T, Hanrath P. Endobronchial sonography: feasibility and preliminary results. Thorax 1992;47:565-7. [Crossref] [PubMed]
- Fernandez-Bussy S, Labarca G, Pires Y, et al. Molecular Testing of EGFR, EGFR Resistance Mutation, ALK and ROS1 Achieved by EBUS-TBNA in Chile. Arch Bronconeumol 2017;53:172-4. [PubMed]
- Righi L, Franzi F, Montarolo F, et al. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA)-from morphology to molecular testing. J Thorac Dis 2017;9:S395-404. [Crossref] [PubMed]
- Yarmus L, Akulian J, Gilbert C, et al. Optimizing endobronchial ultrasound for molecular analysis. How many passes are needed? Ann Am Thorac Soc 2013;10:636-43. [Crossref] [PubMed]
- Jeyabalan A, Bhatt N, Plummeridge MJ, et al. Adequacy of endobronchial ultrasound-guided transbronchial needle aspiration samples processed as histopathological samples for genetic mutation analysis in lung adenocarcinoma. Mol Clin Oncol 2016;4:119-25. [Crossref] [PubMed]
- Sakakibara R, Inamura K, Tambo Y, et al. EBUS-TBNA as a Promising Method for the Evaluation of Tumor PD-L1 Expression in Lung Cancer. Clin Lung Cancer 2017;18:527-534.e1. [Crossref] [PubMed]
- Sakata KK, Midthun DE, Mullon JJ, et al. Comparison of Programmed Death Ligand-1 Immunohistochemical Staining Between Endobronchial Ultrasound Transbronchial Needle Aspiration and Resected Lung Cancer Specimens. Chest 2018;154:827-37. [Crossref] [PubMed]
- Garwood S, Judson MA, Silvestri G, et al. Endobronchial ultrasound for the diagnosis of pulmonary sarcoidosis. Chest 2007;132:1298-304. [Crossref] [PubMed]
- Tremblay A, Stather DR, MacEachern P, et al. A randomized controlled trial of standard vs endobronchial ultrasonography-guided transbronchial needle aspiration in patients with suspected sarcoidosis. Chest 2009;136:340-6. [Crossref] [PubMed]
- Plit M, Pearson R, Havryk A, et al. Diagnostic utility of endobronchial ultrasound-guided transbronchial needle aspiration compared with transbronchial and endobronchial biopsy for suspected sarcoidosis. Intern Med J 2012;42:434-8. [Crossref] [PubMed]
- Hu LX, Chen RX, Huang H, et al. Endobronchial Ultrasound-guided Transbronchial Needle Aspiration versus Standard Bronchoscopic Modalities for Diagnosis of Sarcoidosis: A Meta-analysis. Chin Med J (Engl) 2016;129:1607-15. [Crossref] [PubMed]
- Dziedzic DA, Peryt A, Orlowski T. The role of EBUS-TBNA and standard bronchoscopic modalities in the diagnosis of sarcoidosis. Clin Respir J 2017;11:58-63. [Crossref] [PubMed]
- Moonim MT, Breen R, Fields PA, et al. Diagnosis and subtyping of de novo and relapsed mediastinal lymphomas by endobronchial ultrasound needle aspiration. Am J Respir Crit Care Med 2013;188:1216-23. [Crossref] [PubMed]
- Erer OF, Erol S, Anar C, et al. Diagnostic yield of EBUS-TBNA for lymphoma and review of the literature. Endosc Ultrasound 2017;6:317-22. [Crossref] [PubMed]
- Bandyopadhyay D, Panchabhai TS, Mehta AC. EBUS-TBNA for the Diagnosis of Lymphoma. Still an Achilles Heel. Ann Am Thorac Soc 2015;12:1263-4. [Crossref] [PubMed]
- Oki M, Saka H, Sako C. Bronchoscopic Miniforceps Biopsy for Mediastinal Nodes. J Bronchology 2004;11:150-3. [Crossref]
- Herth FJ, Morgan RK, Eberhardt R, et al. Endobronchial ultrasound-guided miniforceps biopsy in the biopsy of subcarinal masses in patients with low likelihood of non-small cell lung cancer. Ann Thorac Surg 2008;85:1874-8. [Crossref] [PubMed]
- Chrissian A, Misselhorn D, Chen A. Endobronchial-ultrasound guided miniforceps biopsy of mediastinal and hilar lesions. Ann Thorac Surg 2011;92:284-8. [Crossref] [PubMed]
- Franke KJ, Bruckner C, Szyrach M, et al. The contribution of endobronchial ultrasound-guided forceps biopsy in the diagnostic workup of unexplained mediastinal and hilar lymphadenopathy. Lung 2012;190:227-32. [Crossref] [PubMed]
- Herth FJ, Schuler H, Gompelmann D, et al. Endobronchial ultrasound-guided lymph node biopsy with transbronchial needle forceps: a pilot study. Eur Respir J 2012;39:373-7. [Crossref] [PubMed]
- Darwiche K, Freitag L, Nair A, et al. Evaluation of a novel endobronchial ultrasound-guided lymph node forceps in enlarged mediastinal lymph nodes. Respiration 2013;86:229-36. [Crossref] [PubMed]
- Cheng G, Mahajan A, Oh S, et al. EBUS-IFB procedure. Asvide 2019;6:264. Available online: http://www.asvide.com/watch/32949
- Shiari A, Aljundi L, Zein R, et al. Comparing the efficiency of endobronchial ultrasound with the use of microscopic forceps vs fine needle aspiration. Chest 2019;155:263A. [Crossref]
- Bramley K, Pisani MA, Murphy TE, et al. Endobronchial Ultrasound-Guided Cautery-Assisted Transbronchial Forceps Biopsies: Safety and Sensitivity Relative to Transbronchial Needle Aspiration. Ann Thorac Surg 2016;101:1870-6. [Crossref] [PubMed]
- Wolters C, Darwiche K, Franzen D, et al. A Prospective, Randomized Trial for the Comparison of 19-G and 22-G Endobronchial Ultrasound-Guided Transbronchial Aspiration Needles; Introducing a Novel End Point of Sample Weight Corrected for Blood Content. Clin Lung Cancer 2019;20:e265-73. [Crossref] [PubMed]
- Chaddha U, Ronaghi R, Elatre W, et al. Comparison of Sample Adequacy and Diagnostic Yield of 19- and 22-G EBUS-TBNA Needles. J Bronchology Interv Pulmonol 2018;25:264-8. [Crossref] [PubMed]
- Navasakulpong A, Auger M, Gonzalez AV. Yield of EBUS-TBNA for the diagnosis of sarcoidosis: impact of operator and cytopathologist experience. BMJ Open Respir Res 2016;3:e000144. [Crossref] [PubMed]
- Grosu HB, Iliesiu M, Caraway NP, et al. Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration for the Diagnosis and Subtyping of Lymphoma. Ann Am Thorac Soc 2015;12:1336-44. [Crossref] [PubMed]


