
Randomized phase II trial reporting overall survival advantage by adding local consolidative therapy to systemic therapy for oligometastatic non-small cell lung cancer: another step forward on the long road of evidence-based medicine for oligometastatic disease
The term oligometastatic disease (OMD) has been coined in 1995 (1) for patients with a limited number of metastases that may be treated with a potentially curative intention. This intermediate cancer state between localized and metastatic disease was considered rare and both clinical and pre-clinical research was limited. In the recent years, however, OMD gained relevance in particular due to prospective studies highlighting the therapeutic relevance of targeting oligometastatic lesions with locally ablative procedures, such as surgery or stereotactic radiotherapy. Aside from prostate and colorectal cancer (2,3), non-small cell lung cancer (NSCLC) has emerged as one of the major entities of interest. Among the most influential publications on oligometastatic NSCLC is the study by Gomez et al. comparing local consolidative therapy (LCT) and maintenance therapy or observation to maintenance therapy or observation which was first published in 2016 (4). At that time, the authors reported a significantly improved progression-free survival (PFS) and a delay of metastatic progression. In May 2019, the authors provided an update with overall survival (OS) data and additional secondary endpoints (5).
In this randomized, multi-institutional phase II trial, patients with stage IV NSCLC and three or fewer metastases who did not show progression after completing three months of systemic therapy were randomized to either LCT and maintenance systemic therapy/observation or maintenance systemic therapy/observation (MT/O) alone. As recruitment started in 2012, options for systemic therapy included platinum doublet chemotherapy, epidermal growth factor receptor (EGFR)-inhibitors such as erlotinib as well as crizotinib for patients with activating driver mutations. EGFR mutations were present in three patients in each arm (12% respectively) and two patients in the LCT arm (8%) had anaplastic lymphoma kinase (ALK) rearrangement. Immunotherapy was not a component of this study. Due to a very high (99.46%) probability of superiority of the LCT arm in a planned interim analysis of the Data Safety Monitoring Board, the trial was closed early. Median PFS was 14.2 months for patients in the LCT arm compared to 4.4 months in the MT/O arm (log-rank P=0.022). This difference in PFS translated into a difference in OS with a median OS of 42.2 months for patients in the LCT arm compared to 17.0 months in the MT/O arm (P=0.017). While no grade 4 adverse events were observed, grade 3 events occurred in 5 out of 25 patients in the LCT arm (two cases of radiation-induced esophagitis, one case of anemia after radiotherapy to the spleen likely related to treatment, one rib fracture possibly related to treatment, one case of abdominal pain unrelated to treatment) and two out of 24 patients in the MT/O arm (one case of fatigue, one case of anemia).
Limitations in the definition of OMD
An overview of relevant studies on oligometastasis in NSCLC can be found in Table 1. Even though OMD is becoming more and more recognized in daily clinical practice, a clear definition of what exactly constitutes this cancer state is still lacking. Definitions of OMD are currently imaging-based as biomarkers that differentiate between truly oligometastatic patients and patients with diffuse micrometastatic disease are not clinically established. This imaging-based definition gives rise to problems such as the difficulty to compare the results of studies using different imaging modalities or how to deal with increasingly more sensitive novel imaging modalities for patient staging. This shows the need for consensus guidelines of imaging-based diagnosis of OMD as it has been done for example in prostate cancer where standard as well as modern methods such as prostate-specific membrane antigen− (PSMA−) PET are available (8).
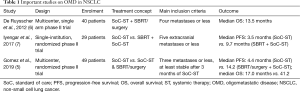
Full table
While the number of metastases has been defined as a criterion in all studies on oligometastatic cancer, the exact number differs and the three or fewer metastases in the Gomez et al.’s study are on the lower end of the spectrum. However, one needs to carefully consider the difference between the maximum number of metastases allowed in a clinical protocol and the actual number of metastases of enrolled patients: for example, the SABR-COMET randomised phase II trial on OMD allowed patients with up to 5 metastases but only 7 of the 99 patients included in the study had more than three (9). Whether the number and the type of involved organ contribute to the definition of OMD is currently unknown.
Additionally, there are more factors beyond the number of metastases that potentially define OMD, influence the prognosis and treatment strategy:
- Did oligometastases occur at the time of primary diagnosis (synchronous) or at later during treatment/follow-ups (metachronous) (10)?
- Do metastatic lymph nodes, in case of NSCLC mediastinal lymph nodes in particular, count towards the total number of metastases (11)?
- How should differences regarding the primary tumor (size, invasion of adjacent structures, response to therapy) be considered?
- Was the patient diagnosis and treated for polymetastatic or OMD before the current diagnosis of OMD?
In addition, other criteria such as the exclusion of untreated intracranial disease (7) renders many studies on oligometastasis difficult to compare. In the Gomez study, an additional criterion is the response to systemic therapy, which is certainly a factor contributing to the exceptional OS.
Selection of the optimal local treatment approach
Studies as the one by Gomez et al. contribute to the paradigm shift towards the importance of local therapy not only for palliation but also to improve survival of patients with OMD. The question of the optimal local treatment modality and its integration into a multimodality treatment concept is therefore highly relevant. The Gomez et al.’s study allowed surgery and radiotherapy or a combination of both: after interdisciplinary discussion, the largest group (48%) of patients was treated with stereotactic body radiation therapy (SBRT) for all lesions and 24% received a combination of surgery and radiotherapy. This supports the importance of SBRT in the oligometastatic setting where the ability to perform the treatment without hospitalisation and tightly integrated into a systemic therapy backbone is highly relevant. However, the pros and cons of all local treatment modalities should be considered on a patient and lesion individual basis: what is the expected toxicity, probability of complete ablation/removal, influence on short and long-term quality of life, associated costs and potential interactions with systemic therapy.
Despite stereotactic radiotherapy being the less invasive procedure and considered a rather safe treatment modality, complications have been described. In addition to the aforementioned toxicities of the Gomez et al.’s study, the SABR-COMET trial noted three treatment-related grade 5 toxicities (one death from radiation pneumonitis, pulmonary abscess and subdural haemorrhage respectively) in the SABR group compared to none in the control group.
Prospective data on a combination of radiotherapy and novel targeted therapies is lacking. However, retrospective data suggests the safety of its application with few combinations where an increased risk has been observed. A potentially increased risk of severe toxicity was noted especially when combining extra-cranial SRT with bevacizumab and sorafenib and cranial SRT with BRAF inhibitors (12).
Whether radiotherapy is delivered concurrently to systemic therapy or whether systemic therapy is paused for a short period of time is another open question. Again, prospective data is missing, but considering the long biochemical and even longer biological half-life of many modern drugs and considering the possible risk of disease flare when the systemic therapy is paused, concurrent treatment appears as the most reasonable strategy (13).
Integration of immune checkpoint inhibition into treatment of OMD
The dominant pattern of disease recurrence after combined modality treatment for OMD is distant with 75–80% of all patients failing. This calls for the integration of more effective systemic therapy into the OMD treatment algorithms. Immune checkpoint inhibition appears to be particularly promising, as this has become the standard of care in first line treatment of polymetastatic NSCLC: either single-agent immune checkpoint inhibition for patients with PD-L1 status of ≥50% or combined chemotherapy and immune checkpoint inhibition (14).
Preclinical data suggest that radiotherapy might be a synergistic partner for the combination with immune checkpoint inhibition (15): radiotherapy can increase leukocyte adhesion to the vascular endothelium (16), upregulate programmed death-ligand 1 (PD-L1) expression on tumor cells (17), cause the release of various inflammatory mediators (18) and affect the tumor microenvironment (19).
The available clinical data support these synergistic effects of radiotherapy and immunotherapy. The PACIFIC study, a randomized phase III trial in unresectable stage III NSCLC, compared consolidation therapy with anti-PD-L1 antibody Durvalumab following definitive radiochemotherapy to placebo (20). For the first time in decades, a significant and clinically relevant improvement of OS was achieved: 24-month OS rate was 66.3% in the durvalumab group, as compared with 55.6% in the control group. And this came without added severe toxicity. A secondary analysis suggested the largest benefit in patients, where Durvalumab was initiated within 14 days after completion of radiotherapy, indicating an interaction between these treatment modalities.
The safety and efficacy of combining immunotherapy and SBRT is also supported by the PEMBRO-RT study. This randomized phase II trial, compared Pembrolizumab alone to Pembrolizumab preceded by SBRT in 3 fractions of 8 Gy to a single metastatic tumor site. The study noted good tolerance of the SBRT/Pembrolizumab combination and an increase in the overall response rate at 12 weeks from 18% in the control arm to 36% in the experimental arm although the predefined criteria for meaningful clinical benefit were not met (21). Especially the PD-L1-negative subgroup showed significant improvement in PFS and OS.
Despite these positive signals, different highly relevant questions need to be addressed:
- What is the optimal timing and sequencing of SBRT and immunotherapy? While performing local therapy first, as it is routinely done in the situation of locally advanced disease, might create an advantage by drastically reducing tumor burden in a short period of time, beginning with systemic therapy could help identifying patients who progress rapidly and therefore will not benefit from local therapy;
- Is there an optimal dose or fractionation scheme of radiotherapy? The evidence as to which dose or scheme creates the strongest abscopal effect, i.e., inducing a systemic effect by irradiating a single tumor site in combination with immunotherapy, is conflicting (22);
- How many lesions should be treated locally? While studies have been initiated trying to create an abscopal effect, others have called to abandon single-site irradiation in favor of treating more, if not all tumor manifestations for optimal results (23);
- Furthermore, additional research is needed on the optimal therapy once a limited number of lesions progress while the patient is still undergoing systemic therapy (oligoprogression). Do patients benefit from adding SBRT to the progressive lesions compared to continuing with systemic therapy alone or should the systemic therapy be changed? One study intending to provide answers to this question for TKI therapy is the HALT trial, a phase II/III multicenter RCT evaluating the addition of SBRT to three or fewer sites of oligoprogressive disease (24).
The ETOP CHESS (NCT03965468) trial will address several of the questions above and test the optimal integration of immunotherapy, chemotherapy, radiotherapy and surgery for synchronous oligometastatic NSCLC: durvalumab, chemotherapy and SBRT to all oligometastatic sites will be combined to maximize local and especially systemic efficacy in an induction phase; this will be followed by radical treatment of the primary, surgery of radiotherapy in unresectable patients. Translational studies will hopefully also shed more light into the biology of OMD.
In conclusion, the updated study by Gomez et al. provides important evidence for the concept of OMD in NSCLC and underlines the necessity to initiate new studies investigating treatment concepts for oligometastatic cancer in the age of immunotherapy.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet 2018;392:2353-66. [Crossref] [PubMed]
- Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309-18; discussion 318-21. [Crossref] [PubMed]
- Gomez DR, Blumenschein GR Jr, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol 2016;17:1672-82. [Crossref] [PubMed]
- Gomez DR, Tang C, Zhang J, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol 2019;37:1558-65. [Crossref] [PubMed]
- De Ruysscher D, Wanders R, van Baardwijk A, et al. Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: long-term results of a prospective phase II trial (Nct01282450). J Thorac Oncol 2012;7:1547-55. [Crossref] [PubMed]
- Iyengar P, Wardak Z, Gerber DE, et al. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol 2018;4:e173501. [Crossref] [PubMed]
- Lecouvet FE, Oprea-Lager DE, Liu Y, et al. Use of modern imaging methods to facilitate trials of metastasis-directed therapy for oligometastatic disease in prostate cancer: a consensus recommendation from the EORTC Imaging Group. Lancet Oncol 2018;19:e534-45. [Crossref] [PubMed]
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 2019;393:2051-8. [Crossref] [PubMed]
- OncologyPRO. Synchronous vs metachronous metastatic disease: Impact of time to metastasis on outcome in metastatic renal cell carcinoma patients treated with targeted therapy. Available online: https://oncologypro.esmo.org/Meeting-Resources/ESMO-2017-Congress/Synchronous-vs-metachronous-metastatic-disease-Impact-of-time-to-metastasis-on-outcome-in-metastatic-renal-cell-carcinoma-patients-treated-with-targeted-therapy
- Giaj-Levra N, Giaj-Levra M, Durieux V, et al. Defining synchronous oligometastatic non-small cell lung cancer: a systematic review. J Thorac Oncol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Kroeze SG, Fritz C, Hoyer M, et al. Toxicity of concurrent stereotactic radiotherapy and targeted therapy or immunotherapy: A systematic review. Cancer Treat Rev 2017;53:25-37. [Crossref] [PubMed]
- Chaft JE, Oxnard GR, Sima CS, et al. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design. Clin Cancer Res 2011;17:6298-303. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373-7. [Crossref] [PubMed]
- Hallahan D, Kuchibhotla J, Wyble C. Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Cancer Res 1996;56:5150-5. [PubMed]
- Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687-95. [Crossref] [PubMed]
- Carvalho HA, Villar RC. Radiotherapy and immune response: the systemic effects of a local treatment. Clinics (Sao Paulo) 2018;73:e557s. [Crossref] [PubMed]
- Barker HE, Paget JTE, Khan AA, et al. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer 2015;15:409-25. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Hu ZI, McArthur HL, Ho AY. The Abscopal Effect of Radiation Therapy: What Is It and How Can We Use It in Breast Cancer? Curr Breast Cancer Rep 2017;9:45-51. [Crossref] [PubMed]
- Brooks ED, Chang JY. Time to abandon single-site irradiation for inducing abscopal effects. Nat Rev Clin Oncol 2019;16:123-35. [Crossref] [PubMed]
- McDonald F, Hanna GG. Oligoprogressive Oncogene-addicted Lung Tumours: Does Stereotactic Body Radiotherapy Have a Role? Introducing the HALT Trial. Clin Oncol (R Coll Radiol) 2018;30:1-4. [Crossref] [PubMed]


