Next-generation care pathways for allergic rhinitis and asthma multimorbidity: a model for multimorbid non-communicable diseases—Meeting Report (Part 2)
Part 2
Introduction and meeting objectives
In all societies, the burden and cost of allergic and chronic respiratory diseases are increasing rapidly. Most economies are struggling to deliver modern health care effectively. There is a need to support the transformation of the health care system into integrated care with organizational health literacy. MASK (Mobile Airways Sentinel NetworK) (1), a new development of the ARIA (Allergic Rhinitis and its Impact on Asthma) initiative (2), and POLLAR (Impact of Air POLLution on Asthma and Rhinitis, EIT Health) (3), in collaboration with professional and patient organizations in the field of allergy and airway diseases, are proposing real-life ICPs—centred around the patient with rhinitis and using mHealth monitoring of environmental exposure.
An expert meeting took place at the Pasteur Institute in Paris, December 3, 2018. The aim was to discuss next-generation care pathways following an ongoing political agenda (4,5): (I) patient participation, health literacy and self-care through technology-assisted “patient activation”; (II) implementation of care pathways by pharmacists and (III) Next-generation guidelines assessing the recommendations of GRADE guidelines in rhinitis and asthma using real-world evidence (RWE) assessed by mobile technology.
The present document reviews the workshop report and follows on from Part 1.
Self-management strategies
Self-management
Self-management may be defined as: “… the tasks that individuals must undertake to live well with chronic conditions. These tasks include having the confidence to deal with the medical management, role management and emotional management of their conditions” (6). Self-management support is the assistance provided by professional/informal caregivers to enable patients to confidently make decisions and manage disease and health-related tasks (7).
The implementation of supported self-management requires patients, professionals and organisations to change behaviour, practice or routines. Behavioural change models, such as COM-B (Capacity, Opportunity, Motivation - Behaviour) (8), offer frameworks for considering implementation strategies, potentially supported by technology.
- People with long-term conditions are, de facto, managing their condition almost all of the time. Professional support aims to empower them to ‘self-manage’ better. Understanding an individual’s beliefs, his/her demographic, cultural and healthcare context, as well as the barriers (such as limited health literacy, poor access to resources) enables an assessment of capacity. Asthma/rhinitis is often a low priority; engaging with self-management support to achieve personal goals may enhance motivation. Technology can conveniently provide reminders, detect triggers, (silently) monitor conditions, provide action plans, keep appointment diaries, facilitate remote consultations and enable peer-to-peer support.
- Professionals’ existing techniques (such as ‘safety-netting’ consultations, providing information) form a basis on which to build the skills of patient-centred consultations (shared decision-making; health coaching), so that asthma/rhinitis supported self-management becomes a normal approach to care. Training needs to motivate (by highlighting benefits to patients and their own professional development) and address individual learning needs (limited experience, de-skilling). Technology can offer computerised decision support and provide on-line learning.
- Organisational routines and local/national healthcare policies can provide opportunities for change but can also stifle innovation. Effective implementation involves tailoring to local capacity, addressing practical barriers, promoting prioritisation of asthma/rhinitis and supported self-management, and reinforcing change. Technology can improve the sharing of (and learning from) data as well as inter-professional communication. It can also help to monitor innovation.
Technological approaches developed with patient/professional stakeholders can underpin many components of supported self-management (9). Self-management ‘apps’ are typically multifaceted, incorporating monitoring of disease status, managing pharmacotherapy, improving adherence, providing reminders, linking to information, and supporting lifestyle changes (10). Consideration of Behavioural change theory (such as COM-B) and practical taxonomies (for example PRISMS) suggests that telehealth could potentially contribute to a broader range of support strategies (11).
The self-management group are developing a 4-page pocket guide on supported self-management (including the use of mobile technology) in the context of the rhinitis integrated care pathway. More widely, they will provide innovative approaches to dissemination.
Systemic changes needed for self-management
Well-developed healthcare cultures and empowered individuals already use self-management as a beacon and tradition. But overall equity and sustainability of our healthcare and societal model (12) require that we strive towards a nurturing process that extends practices of self-care and self-management to all, making self-management the norm rather than the exception.
We therefore point to four areas of systemic change that are needed to benefit this process.
Health literacy
We need to recognize that improved health literacy is the result of strategic adaptations—not just of information material, but also of our educational system (13). We also need to help all citizens to understand symptoms and labels, and empower them for a lifetime of curious and considerate energy to link their natural perseverance with the quality of life they want. Concretely, this would require the school curriculum to teach “prevention” and to slightly shift the teaching of biology, food science, social science, etc. towards a user-need perspective (14).
Labour market
Most of us spend at least a third of our adult life in the labour market. Naturally our actions and conditions in the work place have a significant impact on our health and productivity. Instead of simply considering health promotion in the workplace as a benefit for the employee, both management and research should be shifted towards a holistic productivity perspective (15). Put simply, supporting better health and self-management in employees is obviously good for profits. At system level, it is also obviously as good as SOP in every company, as deficiencies are bad for company profits, bad for national economies, and bad for the health and wellbeing of citizens (16). But let co-creation & incentivization be the primary tool, not simply legislative command & control.
Research
Studying self-management and developing new methods (physical, digital, structures) of support require a wider perspective than that of natural science only. To study health and wellbeing, fields like sociology (17), anthropology, psychology, communication, and law should be included. There should be more emphasis on interdisciplinary research (18) involving support for emerging practices, as this type of prevention is rarely studied and documented.
Policy
Efforts towards sustainability in European policies have shown how joint goals have significant impact, even years before they can be realized. The same is true for self-management. We should realize that to solve certain challenges and reach certain goals in self-management, our policies need to first support these changes (19). Integrating self-management into policy means looking not only at health policies, but also at social, development, educational, research, employment and economic policies (9). If we do so, we can lessen the health inequality, reduce strains on traditional healthcare and ensure an empowering system of change that promotes self-management (20).
Personalised care—supporting self management
There is no universally-agreed definition for personalised care. NHS England defines personalised care as “people having choice and control over the way their care is planned and delivered”. It is based on ‘what matters’ to them and their individual strengths and needs (21). Personalised care is people having choice and control over decisions that affect their own health and wellbeing within a system that harnesses the expertise, capacity and potential of people, families and communities in delivering better outcomes and reducing health inequalities.
In relation to long-term conditions, this means ensuring that people with long-term physical and mental health conditions have the support to build knowledge, skills and confidence to enable them to make informed choices about treatments and to be able to effectively manage their health condition on a day-to-day basis.
Patients with low health literacy should be proactively identified and support should be tailored and targeted in a way that is appropriate for the patient and that takes into account their capability, opportunity and motivation to proactively [COM-B model (8)] manage their health.
Evidence-based types of support may include structured self-management education, health coaching and peer support. New behaviours may then be reinforced and supported daily through Apps and mobile technology.
The integrated care vision of the practicing allergist
Allergists have multiple complex tasks to perform for their patients: they must make clinical diagnoses, perform allergic tests, establish a relationship between the results and the clinical history of the patient. They have to determine the allergic or non-allergic features as well as the non-specific hyperactivity of the clinical signs (22-24).
They also have to explore all cofactors of the allergy, the multimorbidities and their entire environment (25).
They must also implement a therapeutic strategy: symptomatic treatment adapted to the lifestyle of the patient, to his/her personality and to his/her desires.
A relationship of trust should be established rapidly—key to the success of a treatment and a long-term follow-up is required for chronic disease.
Allergic rhinitis is a complex disease that results from interactions between multiple genetic and environmental factors (the exposome). Indoor and outdoor aeroallergens and air pollutants therefore play a key role in the etiopathogenesis of the inflammatory response to allergens and in the clinical manifestations of allergic rhinitis. The allergist may have to help the patient find other allergens, hidden moulds, tobacco smoke, ubiquitous domestic pollutants, sources of volatile/semi-volatile organic compounds (VOCs/SVOCs) (26,27).
The practitioner must deal with all the epithelial barriers of the patient: cutaneous (eczema), digestive (food allergies), eyes, ENT and bronchial (asthma) and needs to determine the therapeutic priorities. Management of the patient’s stressors is essential for a successful treatment and understanding of the patient’s allergy history (28).
The intervention of new technologies will revolutionize the care of the patients: analysis of the indoor environment, establishment of a timeline, follow-up of treatment, allergenic desensitization, therapeutic education. For example, preparation of the next visit, which will consist of reviewing the symptoms that have occurred in the meantime, can be facilitated by the collection of events on the smartphone. This tool may help the allergist to understand the patient and his/her behaviour (1).
ARIA in the pharmacy
The paradigm of how we manage chronic diseases is shifting with a growing understanding that this is a complex process, requiring a coordinated effort from healthcare providers and patients. Pharmacists are key members of these integrated care pathways (ICPs) resolving medication-related problems, optimizing regimens, improving adherence and recommending therapies while establishing liaisons between patients and physicians (2).
More than 15 years after the seminal “ARIA in the Pharmacy” paper (29), much has changed in community pharmacy and allergic rhinitis (AR) management (30,31). Our aim for the session was to develop a Pocket Guide (PG) to be used by pharmacists in the management of AR. This PG provides a clinical guide for pharmacists which is consistent with latest evidence, updated AR guidelines for pharmacy (32) and ICPs as articulated in the latest ARIA AR guidelines.
AR is a complex chronic condition and implementing guidelines for AR management in community pharmacy is particularly important, as most patients do not consult a medical practitioner when selecting medication for their AR. The pharmacist’s role must therefore encompass a broad range of management issues including confirming the presence of AR, treatment selection, patient self-management, long-term monitoring and patient support.
With the high level of self-diagnosis, the ability of pharmacists to recognise AR is critical. The proposed PG includes an 8-item questionnaire to assist the pharmacist and subsequently a flowchart to match the diagnosis to appropriate and optimal treatment for AR. Guidelines consider a range of medications that can be used in the treatment of AR based on symptom severity and duration. Considering non-prescribed medications, intra nasal corticosteroids (INCS) are the most effective treatment for AR, especially in patients with co-existing asthma. Pricing, cultural barriers, specific country regulations, availability, and even patients’ preference for oral vs nasal treatment all mean that INCS may not necessarily be available or the most desirable treatment for all patients. Therefore, a broader understanding of local context was taken into consideration when recommending treatment.
AR control should be considered as the most important endpoint, as it represents a measure of the efficacy of the prescribed treatment and of the patient’s quality of life. In this regard, the proposed flowchart uses Visual Analogue Scales (VASs) to assist pharmacists in determining the optimal non-prescribed medication to recommend. The MASK-air app is proposed for AR monitoring, as this tool appears to be appropriate for most patients and enables patient follow-up (33).
The working group members believe that the integration and shared responsibility of all the stakeholders proposed in a common ICP can (I) improve AR outcome, (II) ensure appropriate, safe and cost-effective medication use, and (III) lower the health care utilisation rate through a more appropriate and timely utilisation of health care services.
Next-generation guidelines
Background
Guidelines are recommendations intended to assist providers and recipients of health care and other stakeholders in making informed decisions. However, single guideline recommendations often require context and integration in care pathways. This is because patients’ problems are often complex and require stepwise, pathway driven approaches to patient care. In turn, this creates problems about the certainty of the evidence in decisions because such decisions should be supported by unbiased evidence.
The methodology for the integration or development of recommendations from pathways is not well defined. Piecing individual recommendations together will often lead to low or very low certainty evidence in the pathway because of missing information for evidence that links steps in a pathway together. This requires rethinking both guideline development and study design.
For instance, the 2016 ARIA guidelines included the following two recommendations (Table 1).
How can such recommendations be integrated? To achieve this, we require study designs that can test the combinations of such recommendations and achieve high certainty in pathways. According to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (35,36), high certainty requires that evidence is at low risk of bias, applicable, consistent (across studies), sufficiently precise and free from publication bias.
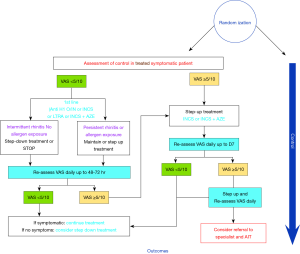
For example, we require studies that evaluate pathways such as those described in Figure 1 to achieve this high certainty of evidence (Figure 1).
We believe that new technology—mHealth (37)—can help with achieving this goal. Randomized trials that provide direct data on care pathways compared to usual care are doable and will help to achieve high certainty in the evidence. Such care will explore linked evidence directly compared to alternative strategies. Furthermore, they can provide widely applicable evidence, often erroneously called “real-world evidence”.
Innovative designs for chronic disease implementation trials
Background
Guideline recommendations often address isolated questions: they frequently focus on a single disease or problem or are not considered in context of the many decisions that are made along the pathway of moving from a health care problem and question to addressing the problem. Care pathways try to address the multiple options and iterative changes in a patient’s status and problems. Guideline recommendations should support these iterative changes.
However, the key challenge is that available evidence, both randomized trials and non-randomized studies, does not usually address the complex pathways and typically addresses the isolated decision points in a pathway. For example, when medication x is not achieving symptom control, we add medication y, then we leave our x and add z. However, often, this is not the way that studies are designed. Accepting that properly developed pathways require evidence, our guidelines must start identifying the best available evidence to support the decisions. The evidence is typically indirect and leads to connecting the relevant decision points. Considering all of the evidence together, the pathway is likely to be supported by low certainty in its overall structure (decision points) and timing.
Approach
The next-generation guidelines, through the intelligent use of tools like ARIA-based apps, in which patients record symptoms and that provide advice at given time points to follow pathways, are a unique opportunity to implement ARIA recommendations and to evaluate them in pathways. Studies should be carried out with patients being randomized to integrated care pathways or to follow ARIA recommendations that are not presented as pathways. Such studies will provide information on the use of the recommendations and on the usefulness of the pathways. Through implementation of recommendations, we will be able to increase our certainty in the evidence by evaluating the entire pathway and measuring outcomes in direct population-based studies that measure what patients do as opposed to what clinicians prescribe (and patients do not do).
Purpose of the meeting
We will present suggestions for the design and use active group discussion to discuss how to use existing data to better design trials, in particular through the use of mobile apps.
Care pathways in allergen immunotherapy
Allergen immunotherapy (AIT) is a proven therapeutic option for the treatment of allergic rhinitis and/or asthma. Many guidelines or national practice guidelines have been produced but the evidence-based method varies, many are complex and none propose care pathways. This paper reviews care pathways for AIT using strict criteria and provides simple recommendations that can be used by all stakeholders including health care professionals. The decision to prescribe AIT for the patient should be individualized and based on the relevance of the allergens, the persistence of symptoms despite appropriate medications according to guidelines as well as on the availability of good-quality and efficacious extracts. Allergen extracts cannot be regarded as generics. Immunotherapy is selected by specialists for stratified patients. There are no currently available validated biomarkers that can predict AIT success. In adolescents and adults, AIT should be reserved for patients with moderate/severe rhinitis or for those with moderate asthma who, despite appropriate pharmacotherapy and adherence, continue to exhibit exacerbations that appear to be related to allergen exposure, except in some specific cases. Immunotherapy may be even more advantageous in patients with multimorbidity. In children, AIT may prevent asthma onset in patients with rhinitis. mHealth tools are promising for the stratification and follow-up of patients.
Deployment of ICPs to other chronic respiratory diseases
Integrated care pathways in asthma
Asthma is a chronic disease characterized by variable symptoms such as shortness of breath, chest tightness and cough, associated with chronic airway inflammation and bronchial hyperresponsiveness. Worldwide, more than 300 million people suffer from asthma and, in 2015, 360,000 patients died due to this disease (38). There is thus a huge need for better treatment and management of asthma in order to alleviate symptoms and prevent asthma attacks (i.e. exacerbations) and mortality. The treatment of asthma encompasses non-pharmacologic approaches such as allergen avoidance (in allergic asthmatics), smoking cessation (in smoking asthmatics) and weight reduction (in obese patients) as well as pharmacologic treatments (39). Asthma drug treatments consist of (I) maintenance treatment with inhaled corticosteroids (ICS) with or without long-acting beta2-agonists (LABA), and (II) rapid-acting reliever therapies used as needed [such as short-acting beta2-agonists (SABA) or fixed combinations of ICS and formoterol 9ICS/form)]. Non-adherence to ICS, overuse of SABA and incorrect inhaler technique are important risk factors for lack of asthma control and life-threatening asthma attacks (40,41).
In asthma, as in other non-communicable chronic diseases (NCDs), several different levels of care can be discerned: patient self-care, care by the pharmacist, (primary) care by general practitioners and allied health professionals, (secondary) care by respiratory physicians and other specialists, and, finally, acute care during emergency department visits and/or during hospital admissions (42). A smooth integration of asthma care between all these levels into integrated care pathways (ICPs) is crucial in order to optimize asthma management and to reduce its burden of disease. Since pharmacists have a frequent contact with asthma patients (when renewing their medications) and have access to previous drug dispensing data, they can play a key role in improving adherence to ICS-containing therapies (as maintenance and/or as reliever), in preventing regular use or abuse of SABA and in optimizing inhaler technique. Patients whose asthma is not well-controlled should be referred promptly to primary care—and, if needed, to secondary care—for proper (differential) diagnosis and management. Secure user-friendly digital platforms should facilitate the integration of the pharmacist dossier and the electronic medical records. Bidirectional e-communication and interaction between all levels of care is required to successfully implement integrated care pathways (ICPs) in asthma in real life.
Care pathways in chronic rhinosinusitis
Care pathways for CRS have been defined in the European position paper on rhinosinusitis and nasal polyps (43) and in a commissioning CRS guide funded by ENT-UK and the Royal College of Surgeons (44). Different algorithms have been developed for primary and secondary care and in secondary care for chronic rhinosinusitis with (CRSwNP) or without (CRSsNP) the presence of nasal polyps. Diagnosis of CRS is based on the presence of 2 or more symptoms of which one should be nasal obstruction or discoloured discharge with or without facial pain, headache or smell disturbance for ≥12 weeks (45). Care pathways are insufficiently described for patient self-management on the one hand and for recalcitrant disease on the other hand (4). Also, co-morbidities are rarely part of the integrated approach for CRS. Especially in relation to new treatment options with monoclonal antibodies, an integrated approach of upper and lower airways is crucial (46).
Moreover, there is a need for continuous re-evaluation and optimization of existing care pathways to increase the level of disease control. Also in CRS, an online educational platform is needed to inform both patients and health care professionals about these issues (45).
Deployment of ICPs to COPD
Chronic Obstructive Pulmonary Disease (COPD) is the third leading cause of death globally. Its prevalence is likely to increase as populations live much longer. The most frequent risk factors for COPD are smoking tobacco and exposure to air pollution, either indoors or outdoors. The majority of individuals with COPD live in low- and middle-income countries (LMIC) and have not been diagnosed (47). While much emphasis must be given to primary prevention, there is an urgent need to promote an earlier diagnosis and management in most countries. There is no cure for COPD. However, secondary preventive measures related to avoiding further damage by stopping smoking and/or reducing exposure to pollution, combined with measures to prevent respiratory infections, a healthy diet, physical activity and medication, can reduce symptoms and exacerbations, improve quality of life and increase survival (47). The use of a mobile phone App may help patients and health care professionals to control COPD, through integrated care pathways, by several means: (I) raising awareness on the condition; (II) performing screening for symptoms of COPD among individuals at risk and suggesting that a doctor should be consulted and spirometry performed, when needed; (III) monitoring symptoms to inform treatment; (IV) early detection of exacerbations; (V) reminders for adherence to treatment; (VI) informing family doctors of exacerbations requiring visits to emergency rooms; (VII) guiding patients on self-management; (VIII) offering information on air pollution; (IX) collecting early information on trends of increasing symptoms and exacerbations, which may alert for preparedness of individuals and health systems. An adaptation of the Allergy Diary, an App developed for allergic rhinitis which has been deployed in over 23 countries (30), may speed up the process of development and deployment. Solutions for common health problems in LMIC must be innovative to combine effectiveness and low cost (48). The use of the proposed App has the potential to support policies aiming to detect COPD earlier, to foster behaviour modification including adherence to treatment, to guide self-management and provide danger alerts (49). The likely synergy of these features may deliver efficiency into overloaded and troublesome health care systems (50).
The paediatric approach
AIT for respiratory allergy is currently used in children with persistent/unresponsive symptoms to treatment (51). Claims for long-term efficacy have been suggested for years (52). However, supporting evidence is weak and long-term intervention studies in children are difficult and even unethical. Although the primary end point of the recent GAP trial was not met, secondary end points suggested a disease-modifying effect on the onset of asthma (53).
AIT is a paradigm for personalised medicine. It takes the multitude of sensitisation and multimorbidity profiles of different patients into account, both cross-sectionally and in relation to their natural history. Indirect yet robust evidence provides clues on patients that may provide more benefit: the severity of respiratory allergic disease is associated with its persistence (54). Epitope spreading and development of new sensitisations suggest benefit with early intervention. Effects on school performance and education (55) further support the need for maximisation of treatment at a time of developmental/career milestones.
In parallel, more studies are needed to characterise long-term effects. Such studies cannot be randomised and even less blinded. Therefore, observational approaches, such as registry research, need to be used (56). Criteria for the quality assessment of such approaches are already available and rapidly developing (Roche, Papadopoulos et al., in press).
In addition, there are opportunities for disease prevention that have not been adequately explored, such as primary prevention. Support for such studies needs to come from governmental organisations/public sources, in order to identify optimal cost-efficacy strategies.
Deployment to developing countries
Chronic respiratory diseases (CRDs) affect more than one billion people and everyone is exposed to risk factors (57). They are inter-related to infectious conditions, which often exacerbate CRDs or may cause them. Common colds are the most frequent trigger of asthma and COPD exacerbations, whereas various other respiratory infections, including RSV, tuberculosis and HIV, may induce CRD. Respiratory diseases are among the leading causes of death globally (47). At the other side of the spectrum, in primary health care (PHC), respiratory diseases are the most frequent cause of medical visits (58). The burden of these diseases is disproportionally higher in LMIC, where diagnosis and management may be late. The cost of health is increasing with longevity, novel technologies for diagnosis and treatments, and CRDs are associated with multimorbidity. Fragmented and/or specialized approaches to PHC are inefficient and costly. An integrated care pathway to CRD has been proposed as a way to change current management strategies (2).
The challenge of LMIC to offer good quality universal PHC demands innovative solutions. In this context, a change in current paradigms to take advantage of mobile communications technology and give patients a prominent role in their health care is an opportunity not to be missed. The model of the Allergy Diary, an App developed for rhinitis deployed in over 23 countries (30,59), may facilitate this. A pilot experience for a new App aimed at people at risk of COPD primed by large media campaigns for raising recognition is proposed to be undertaken in Turkey, Vietnam and Brazil by leaders of the Global Alliance against Chronic Respiratory Diseases (GARD)/WHO (60). The objectives of this COPD App plan are: (I) raise awareness of COPD and risk factors; (II) screen subjects at risk by a simple electronic questionnaire; (III) suggest that people with scores indicative of COPD see a doctor and perform spirometry; (IV) estimate the proportion of under diagnosis; (V) monitor symptoms to inform treatment; (VI) detect exacerbations early. Three steps are required for this plan to be rolled out: (I) invite GARD Country organizations, including the Ministry of Health, to discuss (6 months); (II) search for local funding for the campaign (1 year); (III) define an action plan and roll it out, partnering with as many organizations as possible, including governmental organizations, associations of health care professionals, universities and the private sector (18 months).
Acknowledgments
We would like to thank the following companies for their unrestricted educational grants: Mylan, ALK, GSK, Novartis, Sanofi, Stallergenes-Greer, Uriach.
Footnote
Conflicts of Interest: Dr. Ansotegui reports personal fees from Mundipharma, Roxall, Sanofi, MSD, Faes Farma, Hikma, UCB, Astra Zeneca, outside the submitted work. Dr. Bachert reports personal fees from ALK, Stallergen, during the conduct of the study; personal fees from ALK, Stallergen, outside the submitted work. Dr. Bousquet reports personal fees from Chiesi, Cipla, Hikma, Menarini, Mundipharma, Mylan, Novartis, Purina, Sanofi-Aventis, Takeda, Teva, Uriach, other from KYomed-Innov, outside the submitted work. Dr. Calderon reports personal fees from ALK-Abello, ALK-US, Stallergenes Greer, HAL-Allergy, Allergopharma, ASIT-Biotech, outside the submitted work. Dr. Canonica reports grants from ALK ABELLO, Allergy Therapeutics, Anallergo, Hal Allergy, Stallergenes Greer, outside the submitted work. Dr. Cardona reports personal fees from ALK, Allergopharma, Allergy Therapeutics, Diater, LETI, Thermofisher, Stallergenes, outside the submitted work. Dr. Cecchi reports personal fees from Menarini, Malesci ALK, outside the submitted work. Dr. Cruz reports grants from National Institutes for Health Research (UK), National Institutes of Health (USA), grants and other from National Research Council (Brazil), other from Federal University of Bahia (Brazil), non-financial support from Fundacao ProAR, grants and personal fees from GSK, personal fees from AstraZeneca, Boehringer Ingelheim, CHIESI, Eurofarma, MEDA Pharma. Dr. Durham reports personal fees from Adiga, personal fees from ALK, personal fees from Allergopharma, MedicalUpdate GmBC, UCB, outside the submitted work. Dr. Ebisawa reports personal fees from Mylan, DBV Technologies, Thermofisher, outside the submitted work. Dr. Fokkens reports grants from Mylan, Allergy Therapeutics, GSK, ALK. Dr. Fonseca being a partner in a company developing mobile technologies for monitoring airways diseases. Dr. Klimek reports grants and personal fees from ALK Abelló, Denmark, grants and personal fees from Novartis, Switzerland, Allergopharma, Germany, Bionorica, Sweden, GSK, Great Britain, Lofarma, Italy, personal fees from MEDA, Sweden, Boehringer Ingelheim, Germany, grants from Biomay, Austria, grants from HAL, Netherlands, grants from LETI, Spain, Roxall, Germany, Bencard, Great Britain, outside the submitted work. Dr. Kuna reports personal fees from Adamed, AstraZeneca, Boehringer Ingelheim, Hal, Chiesi, Novartis, Berlin Chemie Menarini, outside the submitted work. Dr. Kvedariene reports personal fees from GSK, non-financial support from StallergenGreer, Mylan, AstraZeneca, Dimuna, Norameda, outside the submitted work. D Larenas Linnemann reports personal fees from GSK, Astrazeneca, MEDA, Boehringer Ingelheim, Novartis, Grunenthal, UCB, Amstrong, Siegfried, DBV Technologies, MSD, Pfizer. grants from Sanofi, Astrazeneca, Novartis, UCB, GSK, TEVA, Chiesi, Boehringer Ingelheim, outside the submitted work. Dr. MULLOL reports personal fees from SANOFI-GenzymeRegeneron, ALK-Abelló A/S, Menarini Group, MSD, GlaxoSmithKline, Novartis, GENENTECH-Roche, grants and personal fees from UCB Pharma, MYLAN-MEDA Pharma, URIACH Group, outside the submitted work. Y Okamoto reports personal fees from Shionogi Co. Ltd., Torii Co. Ltd., GSK, MSD, Kyowa Co. Ltd., from Eizai Co. Ltd., grants and personal fees from Kyorin Co. Ltd., Tiho Co. Ltd., grants from Yakuruto Co. Ltd., Yamada Bee Farm, outside the submitted work. N Papadopoulos reports personal fees from Novartis, Faes Farma, BIOMAY, HAL, Nutricia Research, Menarini, Novartis, MEDA, Abbvie, Novartis, MSD, Omega Pharma Danone, grants from Menarini outside the submitted work. O Pfaar reports grants and personal fees from ALK-Abelló, Allergopharma, Stallergenes Greer, HAL Allergy Holding B.V./HAL Allergie GmbH, Bencard Allergie GmbH/Allergy Therapeutics, Lofarma, Biotech Tools S.A, LETI/LETI Pharma, Anergis S.A. grants from Biomay, Nuvo, Circassia, Glaxo Smith Kline, personal fees from Novartis Pharma, MEDA Pharma, Mobile Chamber Experts (a GA2LEN Partner), Pohl-Boskamp, Indoor Biotechnologies, grants from, outside the submitted work. Dr. Samolinski reports non-financial support from Mylan, during the conduct of the study. Dr. Shamji reports grants and personal fees from ALK, ASIT Biotech, sa, Allergopharma, grants from Regeneron, Merck, Immune Tolerance Network, outside the submitted work. Dr. Tsiligianni reports personal fees from Novartis, GSK, Boehringer Ingelheim, Astra Zeneca, grants from GSK Hellas, outside the submitted work. Dr. Wallace is co-chair of the Joint Task Force on Practice Parameters of the AAAAI/ACAAI. However, it does not feel that this causes any conflict of interest in the writing/review of the document. Zuberbier reports fees from Bayer Health Care, FAES, Novartis, Henkel, Astra Zeneca, AbbVie, ALK, Almirrall, Astellas, Bayer Health Care, Bencard, Berlin Chemie, HAL, Leti, Meda, Menarini, Merck, MSD, Pfizer, Sanofi, Stallergenes, Takeda, Teva, UCB, Henkel, Kryolan, l’Oreal; Commitee member: WHO-Initiative “Allergic Rhinitis and Its Impact on Asthma” (ARIA). Member of the Board: German Society for Allergy and Clinical Immunology (DGAKI). Head: European Centre for Allergy Research Foundation (ECARF). Secretary General: Global Allergy and Asthma European Network (GA2LEN). Member: Committee on Allergy Diagnosis and Molecular Allergology, World Allergy Organization (WAO). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Bousquet J, Arnavielhe S, Bedbrook A, et al. MASK 2017: ARIA digitally-enabled, integrated, person-centred care for rhinitis and asthma multimorbidity using real-world-evidence. Clin Transl Allergy 2018;8:45. [Crossref] [PubMed]
- Bousquet J, Hellings PW, Agache I, et al. ARIA Phase 4 (2018): Change management in allergic rhinitis and asthma multimorbidity using mobile technology. J Allergy Clin Immunol 2018. [Crossref] [PubMed]
- Bousquet J, Anto JM, Annesi-Maesano I, et al. POLLAR: Impact of air POLLution on Asthma and Rhinitis; a European Institute of Innovation and Technology Health (EIT Health) project. Clin Transl Allergy 2018;8:36. [Crossref] [PubMed]
- Hellings PW, Borrelli D, Pietikainen S, et al. European Summit on the Prevention and Self-Management of Chronic Respiratory Diseases: report of the European Union Parliament Summit (29 March 2017). Clin Transl Allergy 2017;7:49. [Crossref] [PubMed]
- Valiulis A, Bousquet J, Veryga A, et al. Vilnius Declaration on chronic respiratory diseases: multisectoral care pathways embedding guided self-management, mHealth and air pollution in chronic respiratory diseases. Clin Transl Allergy 2019;9:7. [Crossref] [PubMed]
- Adams K, Greiner A, JM JC. The 1st Annual Crossing the Quality Chasm Summit – A Focus on Communities Chapter 5. The National Academic Press, Washington, D.C. 2004. 2004.
- de-Silva D. Helping people help themselves: A review of the evidence considering whether it is worthwhile to support self-management. The Health Foundation. Available online: (Accessed December 2018) 2011.https://www.health.org.uk/sites/default/files/HelpingPeopleHelpThemselves.pdf
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6:42. [Crossref] [PubMed]
- Pearce G, Parke H, Pinnock H, et al. The PRISMS Taxonomy of Self-Management Support: Derivation of a Novel Taxonomy and Initial Testing of Utility. J Health Serv Res Policy 2016;21:73-82. [Crossref] [PubMed]
- Hui CY, Walton R, McKinstry B, et al. The use of mobile applications to support self-management for people with asthma: a systematic review of controlled studies to identify features associated with clinical effectiveness and adherence. J Am Med Inform Assoc 2017;24:619-32. [PubMed]
- Pinnock H, Effing T, Bourbeau J, et al. Self-management of respiratory disease. Respipedia, the respiratory wiki. 29.09.2017 04:28. Available online: http://respipedia.ers-education.org/article/article/?idTopic=217. 2017.
- PiSCE Final Report. Available online: 2017.www.selfcare.nu
- National Action Plan on Health Literacy in Germany. Available online: 2018:28-9.https://www.nap-gesundheitskompetenz.de/
- Rajah R, Ahmad Hassali MA, Jou LC, et al. The perspective of healthcare providers and patients on health literacy: a systematic review of the quantitative and qualitative studies. Perspect Public Health 2018;138:122-32. [Crossref] [PubMed]
- Pescud M, Teal R, Shilton T, et al. Employers' views on the promotion of workplace health and wellbeing: a qualitative study. BMC Public Health 2015;15:642. [Crossref] [PubMed]
- Havner M, van-Stolk C, Saunders C, et al. Health, wellbeing and productivity in the workplace. A Britain's Healthiest Company summary report" RAND Corporation Report, Available online: 2015.https://www.rand.org/pubs/research_reports/RR1084.html
- Lin YK, Hsu MT, Hsieh MC. Anthropological and sociological perspectives of medical professionalism. Ci Ji Yi Xue Za Zhi 2018;30:53-4. [PubMed]
- Gullov E. Institutionalizd Visions for a Good Life in Danish Day-care Centres. Anthropol Action 2011;18:21-32. [Crossref]
- Duncan B. Health policy in the European Union: how it's made and how to influence it. BMJ 2002;324:1027-30. [Crossref] [PubMed]
- Hedbderg A, Hines P. Addressing the crisis of tomorrow: the sustainability of European health systems" European Policy Centre, Policy Brief. Available online: 2016.https://www.epc.eu/pub_details.php?cat_id=3&pub_id=6951
- personalized care. NHS England; Available online: https://www.england.nhs.uk/personalisedcare/.
- Bachert C, Bousquet J, Hellings P. Rapid onset of action and reduced nasal hyperreactivity: new targets in allergic rhinitis management. Clin Transl Allergy 2018;8:25. [Crossref] [PubMed]
- Hellings PW, Klimek L, Cingi C, et al. Non-allergic rhinitis: Position paper of the European Academy of Allergy and Clinical Immunology. Allergy 2017;72:1657-65. [Crossref] [PubMed]
- Van Gerven L, Steelant B, Hellings PW. Nasal hyperreactivity in rhinitis: A diagnostic and therapeutic challenge. Allergy 2018;73:1784-91. [Crossref] [PubMed]
- Cingi C, Gevaert P, Mosges R, et al. Multi-morbidities of allergic rhinitis in adults: European Academy of Allergy and Clinical Immunology Task Force Report. Clin Transl Allergy 2017;7:17. [Crossref] [PubMed]
- Cecchi L, D'Amato G, Annesi-Maesano I. External exposome and allergic respiratory and skin diseases. J Allergy Clin Immunol 2018;141:846-57. [Crossref] [PubMed]
- Caillaud D, Leynaert B, Keirsbulck M, et al. Indoor mould exposure, asthma and rhinitis: findings from systematic reviews and recent longitudinal studies. Eur Respir Rev 2018;27. [Crossref] [PubMed]
- Oland AA, Booster GD, Bender BG. Integrated behavioral health care for management of stress in allergic diseases. Ann Allergy Asthma Immunol 2018;121:31-6. [Crossref] [PubMed]
- ARIA in the pharmacy: management of allergic rhinitis symptoms in the pharmacy. Allergic rhinitis and its impact on asthma. Allergy 2004;59:373-87. [Crossref] [PubMed]
- Bousquet J, Hellings PW, Agache I, et al. ARIA 2016: Care pathways implementing emerging technologies for predictive medicine in rhinitis and asthma across the life cycle. Clin Transl Allergy 2016;6:47. [Crossref] [PubMed]
- Wise SK, Lin SY, Toskala E, et al. International Consensus Statement on Allergy and Rhinology: Allergic Rhinitis. Int Forum Allergy Rhinol 2018;8:108-352. [PubMed]
- Bosnic-Anticevich S, Costa E, Menditto E, et al. ARIA pharmacy 2018 "Allergic rhinitis care pathways for community pharmacy": AIRWAYS ICPs initiative (European Innovation Partnership on Active and Healthy Ageing, DG CONNECT and DG Santé) POLLAR (Impact of Air POLLution on Asthma and Rhinitis) GARD Demonstration project. Allergy 2019;74:1219-36. [PubMed]
- Caimmi D, Baiz N, Tanno LK, et al. Validation of the MASK-rhinitis visual analogue scale on smartphone screens to assess allergic rhinitis control. Clin Exp Allergy 2017;47:1526-33. [Crossref] [PubMed]
- Brozek JL, Bousquet J, Agache I, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) Guidelines - 2016 Revision. J Allergy Clin Immunol 2017;140:950-8. [Crossref] [PubMed]
- Brozek JL, Akl EA, Compalati E, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines part 3 of 3. The GRADE approach to developing recommendations. Allergy 2011;66:588-95. [Crossref] [PubMed]
- Schunemann HJ, Mustafa R, Brozek J, et al. GRADE Guidelines: 16. GRADE evidence to decision frameworks for tests in clinical practice and public health. J Clin Epidemiol 2016;76:89-98. [Crossref] [PubMed]
- Bousquet J, Chavannes NH, Guldemond N, et al. Realising the potential of mHealth to improve asthma and allergy care: how to shape the future. Eur Respir J 2017.49. [PubMed]
- Collaborators GBDCRD. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med 2017;5:691-706. [Crossref] [PubMed]
- Reddel HK, Bateman ED, Becker A, et al. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J 2015;46:622-39. [Crossref] [PubMed]
- Suissa S, Ernst P, Boivin JF, et al. A cohort analysis of excess mortality in asthma and the use of inhaled beta-agonists. Am J Respir Crit Care Med 1994;149:604-10. [Crossref] [PubMed]
- Suissa S, Ernst P, Benayoun S, et al. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med 2000;343:332-6. [Crossref] [PubMed]
- Hellings PW, Fokkens WJ, Bachert C, et al. Positioning the principles of precision medicine in care pathways for allergic rhinitis and chronic rhinosinusitis - A EUFOREA-ARIA-EPOS-AIRWAYS ICP statement. Allergy 2017;72:1297-305. [Crossref] [PubMed]
- Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012;50:1-12. [Crossref] [PubMed]
- ENT UK commissioning guidelines in collaboration with RCS: Commissioning guide for Rhinosinusitis. Available online: 2016.https://www.entuk.org/commissioning-guides
- Seys SF, Bousquet J, Bachert C, et al. mySinusitisCoach: patient empowerment in chronic rhinosinusitis using mobile technology. Rhinology 2018;56:209-15. [PubMed]
- Hellings PW, Akdis CA, Bachert C, et al. EUFOREA Rhinology Research Forum 2016: report of the brainstorming sessions on needs and priorities in rhinitis and rhinosinusitis. Rhinology 2017;55:202-10. [Crossref] [PubMed]
- Forum of International Respiratory Societies. The Global Impact of Respiratory Disease – Second Edition. Sheffield (UK): European Respiratory Society; 2017.
- Cruz AA, Camargos PA, Urrutia-Pereira M, et al. Global Alliance against Chronic Respiratory Diseases (GARD) Brazil success case: overcoming barriers. J Thorac Dis 2018;10:534-8. [Crossref] [PubMed]
- To T, Cruz AA, Viegi G, et al. A strategy for measuring health outcomes and evaluating impacts of interventions on asthma and COPD-common chronic respiratory diseases in Global Alliance against Chronic Respiratory Diseases (GARD) countries. J Thorac Dis 2018;10:5170-7. [Crossref] [PubMed]
- Time to deliver: report of the WHO Independent High-Level Commission on Noncommunicable Diseases. World Health Organization 2018; Available online: https://www.who.int/ncds/management/time-to-deliver/en/.
- Wilson DR, Torres LI, Durham SR, et al. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev 2003.CD002893. [PubMed]
- Valovirta E, Petersen TH, Piotrowska T, et al. Results from the 5-year SQ grass sublingual immunotherapy tablet asthma prevention (GAP) trial in children with grass pollen allergy. J Allergy Clin Immunol 2018;141:529-38.e13. [Crossref] [PubMed]
- Tai A, Tran H, Roberts M, et al. Outcomes of childhood asthma to the age of 50 years. J Allergy Clin Immunol 2014;133:1572-8.e3. [Crossref] [PubMed]
- Lau S, Matricardi PM, Wahn U, et al. Allergy and atopy from infancy to adulthood: Messages from the German birth cohort MAS. Ann Allergy Asthma Immunol 2019;122:25-32. [Crossref] [PubMed]
- Walker S, Khan-Wasti S, Fletcher M, et al. Seasonal allergic rhinitis is associated with a detrimental effect on examination performance in United Kingdom teenagers: case-control study. J Allergy Clin Immunol 2007;120:381-7. [Crossref] [PubMed]
- Price D, Bateman ED, Chisholm A, et al. Complementing the randomized controlled trial evidence base. Evolution not revolution. Ann Am Thorac Soc 2014;11 Suppl 2:S92-8. [Crossref] [PubMed]
- Bousquet J, Khaltaev N. Global surveillance, prevention and control of Chronic Respiratory Diseases. A comprehensive approach. Global Alliance against Chronic Respiratory Diseases. World Health Organization. ISBN 978 92 4 156346 8 2007:148 pages.
- Martins P, Rosado-Pinto J, do Ceu Teixeira M, et al. Under-report and underdiagnosis of chronic respiratory diseases in an African country. Allergy 2009;64:1061-7. [Crossref] [PubMed]
- Bousquet J, Onorato GL, Bachert C, et al. CHRODIS criteria applied to the MASK (MACVIA-ARIA Sentinel NetworK) Good Practice in allergic rhinitis: a SUNFRAIL report. Clin Transl Allergy 2017;7:37. [Crossref] [PubMed]
- Bousquet J, Mohammad Y, Bedbrook A, et al. Country activities of Global Alliance against Chronic Respiratory Diseases (GARD): focus presentations at the 11th GARD General Meeting, Brussels. J Thorac Dis 2018;10:7064-72. [Crossref] [PubMed]