
Diagnostic approach to chronic dyspnoea in adults
Introduction
Dyspnoea, or breathlessness, is a common symptom in adults presenting to both primary and tertiary care and can often present as a diagnostic challenge (1,2). Dyspnoea is typically classified as chronic when it is present for at least four to eight weeks (1,2). The prevalence of chronic dyspnoea is difficult to ascertain, due to studies reporting both acute and chronic dyspnoea, but it has been reported to affect between 9% and 59% of the general population (3,4). A higher prevalence of chronic dyspnoea has been reported in individuals over 70 years of age, with dyspnoea in these patients being associated with worsening functional decline and increased five year mortality (5,6). Of concern, analysis of 356,799 responders to the online British Lung Foundation Breath Test found that 58% of people who had sought help from their health care providers indicated that the advice given had not helped their breathlessness (7).
Chronic dyspnoea is most commonly due to a respiratory or cardiac cause; however, there is a wide differential diagnosis (8,9) (Table 1). Additionally, the aetiology of chronic dyspnoea is multifactorial in approximately one third of cases, with common co-existing conditions contributing to symptomatology (1,10). The most common causes of chronic dyspnoea include chronic obstructive pulmonary disease (COPD), cardiac failure, asthma, ischaemic heart disease, interstitial lung disease (ILD) and psychological conditions (2,8) (Table 1).

Full table
Barriers to diagnosis include difficulty in selecting the most appropriate investigations and the correct speciality referral for further diagnostic assessment (3,10). For instance, a study has reported that only 51% of patients with chronic dyspnoea, who were referred to cardiology or respiratory clinics at a tertiary hospital, were appropriately referred to the speciality clinic pertaining to their final diagnosis (11). This difficulty in initial referral can lead to significant delays in diagnosis, particularly as chronic dyspnoea diagnosis can take considerable time if more specialised testing is required. Furthermore, studies have reported that less than 50% of primary care diagnoses are concordant with final specialist diagnoses after referral, reflecting the current difficulty in diagnosis (10,11). Additionally, in a subset of patients, a cause may never be identified despite considerable evaluation at a tertiary level (11,12). This is a major issue, since breathlessness is a symptom and effective management relies foremost on diagnosis of the underlying cause of the breathlessness.
Therefore, this review aims to: (I) demonstrate the current clinical difficulties in the diagnosis of patients with chronic dyspnoea; (II) evaluate the diagnostic workup of chronic dyspnoea, with an emphasis on clinical assessment and the utility of investigations; and (III) evaluate current diagnostic algorithms for chronic dyspnoea in adults. A search was undertaken on the MEDLINE, Embase and CINAHL databases from 1985 to 2019 using search terms of ‘dyspnoea’, ‘chronic’, ‘algorithm’ and ‘diagnosis’. Of 278 publications retrieved, 26 publications were assessed to be relevant and included in this literature review. A further 21 papers from additional targeted searches were also included.
Clinical assessment
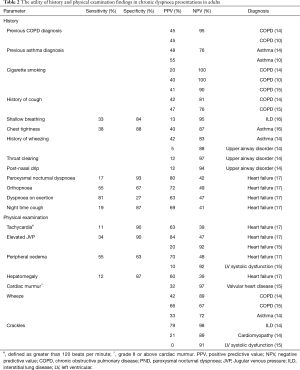
The initial clinical assessment of patients presenting with chronic dyspnoea directs further investigations, specialist referrals if needed, and management. The literature suggests that limitations in the utility of history combined with non-specific examination findings contribute to the difficulty in diagnosis of the cause of chronic dyspnoea in adults (1,13). The majority of history and physical examination findings individually have low positive predictive value (PPV) and are more useful as negative predictive factors (Table 2). However, as clinical findings are rarely considered in isolation, combinations of findings are more likely to confer greater diagnostic accuracy (8,18). In a prospective observational study of 85 patients referred to a specialist pulmonary clinic, clinical evaluation (history and examination) was reported to reach a definitive diagnosis in 66% of chronic dyspnoea presentations (14). However, there are limited studies in chronic dyspnoea populations and as such, these results have not been replicated.
Full table
History
Medical history
Whilst taking a directed history is important in any comprehensive assessment of patients, a patient’s previous medical history may not necessarily be highly predictive of the underlying cause of chronic dyspnoea, which can be multifactorial in aetiology. For example, studies have shown that a previous diagnosis of asthma or COPD has a PPV of approximately 45% for these diagnoses being the cause of a patient’s dyspnoea (13,14). A history of cigarette smoking had a high negative predictive value (NPV) of 90% to 100% for a COPD diagnosis but a low PPV, reflecting the common causality of smoking for numerous cardiorespiratory conditions that lead to chronic dyspnoea (13-15).
Cardiorespiratory symptoms
Overall, common respiratory symptoms with high prevalence such as cough and sputum production generally conferred low specificity for respiratory diagnoses in clinical studies (Table 2). For example, the absence of a cough strongly suggested against a diagnosis of COPD but was an otherwise non-specific symptom (14,15). Upper respiratory tract symptoms such as throat clearing and post-nasal drip were reported to have a high NPV for upper airway disorders in those presenting with chronic dyspnoea (14). Symptoms of orthopnoea and paroxysmal nocturnal dyspnoea were predictive of cardiac failure, with a PPV of 72% and 80%, respectively in an population of patients hospitalised with dyspnoea (17,19).
Dyspnoea quality
The historical description of dyspnoea is important in diagnostic considerations. A study of descriptors of dyspnoea by patients referred to a pulmonary clinic identified that chest tightness was associated with an asthma diagnosis (specificity 88%), and shallow breathing was associated with ILD (specificity 84%) (16). In addition, standardised dyspnoea scales may be utilised to quantify dyspnoea severity. This may have diagnostic implications, with an increased dyspnoea severity reported to increase the likelihood of a COPD diagnosis (20). Similarly, it is important to differentiate dyspnoea from exercise intolerance.
Physical examination
Signs of cardiac failure
Clinically, the detection of multiple physical signs of cardiac failure confers the greatest diagnostic accuracy (21). However, when analysed individually, the utility is reduced, with an elevated JVP (LR+ 3.4) or peripheral oedema (LR+ 1.5) conferring modest likelihood ratios in the chronic dyspnoea setting (17). Furthermore, there is conflicting data for these signs reported in the literature (Table 2). Overall, the best predictors of heart failure are clinical findings that reflect severe decompensated disease, whilst early clinical diagnosis is more challenging (21,22).
Findings on auscultation
On auscultation, a wheeze is not pathognomonic for any particular disease but has a high NPV for both asthma and COPD in the chronic dyspnoea setting (13,15,19). For a diagnosis of ILD, crackles on auscultation were reported to have a 79% PPV and 98% NPV, in a study of patients referred to a specialist pulmonary clinic (14). These fine basal inspiratory crackles have been described to occur in more than 90% of ILD cases and be present early in the disease process (23).
With regards to cardiac disease, an absence of crackles on auscultation suggested against a heart failure diagnosis (NPV 89–91%) but when present this was a non-specific finding (14,15). Similarly, cardiac murmurs are a highly sensitive sign for valvular heart disease, recording a NPV of 97%, but with low specificity when present (15).
Collectively, these findings on history and examination exemplify the generally low PPV of individual clinical findings in chronic dyspnoea diagnoses, and their higher NPV (i.e., if the finding is absent, then the condition is less likely to be present).
Primary investigations
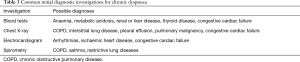
After clinical assessment, a number of initial diagnostic investigations may be considered in the diagnostic workup of the patient with chronic dyspnoea (Table 3). Although the individual utility of these initial investigations varies for specific chronic dyspnoea diagnoses, these are commonly available tests that are relatively easy to perform or request.
Full table
Blood tests
A number of laboratory investigations are commonly utilised in the diagnostic workup of chronic dyspnoea patients and are usually ordered early in this process (1,8). Firstly, a full blood count is useful for checking the haemoglobin level for anaemia, which may cause dyspnoea, or polycythaemia, which may indicate chronic hypoxaemia (8). In one study of chronic dyspnoea patients, haemoglobin was found to be low in 14% of subjects, with dyspnoea improving once haemoglobin was corrected to above 100 g/L (13). Thyroid function tests and biochemistry may not necessarily have a significant impact on diagnosis (13,15), but since thyroid dysfunction or metabolic abnormalities are potential causes of chronic dyspnoea, and these tests are straightforward, they are currently recommended in the diagnostic process (1,2,8).
B-type natriuretic peptide (BNP) is a blood test that detects cardiomyocyte strain and is often used to differentiate between cardiac and respiratory causes of dyspnoea where there is clinical uncertainty (24). BNP is recommended in many heart failure guidelines as a first line test in the diagnosis of heart failure (22,25,26). BNP has been reported as a less expensive and more accessible diagnostic test for heart failure than echocardiography (24). In studies of chronic dyspnoea, the addition of BNP testing has improved diagnostic accuracy and time to diagnosis for cardiomyopathy-related disease (13,27). However, the limitations include uncertainty regarding the most appropriate abnormal cut-off point for the test, with sensitivity and specificity varying depending on the selected threshold (28-30).
Chest radiography
Chest radiography is a valuable tool which has been proposed as a first line investigation after clinical assessment in chronic dyspnoea presentations (14,31). This is due to the number of cardiorespiratory conditions identifiable on chest X-ray (13,15). In suspected cardiac failure, chest X-ray is a useful initial investigation that may show signs of pulmonary oedema or an increased cardiothoracic ratio; however, a patient with cardiac failure need not have pulmonary oedema to have significant symptoms (17,31,32). In chronic dyspnoea studies, chest radiography was found to be most useful for identifying ILD, COPD and cardiac failure, although notably, a significant proportion of patients with asthma and COPD had normal chest X-rays (13,15).
Electrocardiography
An electrocardiogram (ECG) is a readily accessible investigation to detect abnormalities that may suggest underlying cardiac disease causing a patient’s dyspnoea (8). This was emphasised in one study of chronic dyspnoea patients where 8% were found to have atrial fibrillation on ECG, with 80% of these patients later being diagnosed with an underlying cardiac disease that was responsible for their dyspnoea (15). ECGs have been found to have a high NPV (up to 95%) for cardiac conditions, indicating their potential for an early rule out test. However, due to their low specificity, follow-up testing, often with an echocardiogram or other investigations, is required to further define cardiac disease (33,34).
Spirometry
Spirometry is useful for detecting obstructive and restrictive ventilatory patterns which can be further defined on advanced respiratory function testing (35). Similar to chest radiography, it has been suggested as an initial diagnostic tool, especially when airway diseases such as COPD or asthma are suspected (15). In a primary care study of chronic dyspnoea presentations, diagnostic accuracy increased from 55% with clinical assessment to 72% after spirometry findings were considered (13). Another study of chronic dyspnoea identified that 33% of the study population had abnormal spirometry leading to a diagnosis (15). However, spirometry has been reported to be underused in primary care due to lack of time, resources and staff expertise to undertake spirometry (36).
Secondary investigations
After initial investigations, clinicians may consider further specialised investigations or referral to a specialist in tertiary care with a view to additional specialised testing.
Advanced respiratory function tests
Advanced respiratory function tests are a useful adjunct in the assessment of chronic dyspnoea of suspected respiratory origin. When utilised with clinical assessment, lung volume measurements such as residual volume and total lung capacity can further define lung restriction or hyperinflation (8,9). Another key aspect is the measurement of the diffusing capacity of the lung for carbon monoxide (DLCO) (35). In one study of chronic dyspnoea patients, DLCO results were reduced in 19% of patients with otherwise normal spirometry, including two patients with a final diagnosis of left ventricular dysfunction (15). While in another study of patients referred to a pulmonary specialist clinic, DLCO was found to be reduced in 64%, in those receiving a COPD or ILD diagnosis (13).
Bronchoprovocation testing for bronchial hyper-responsiveness has a role in diagnosing asthma in patients with chronic dyspnoea and suspected asthma but normal spirometry (13). In one study, 34% of patients with chronic dyspnoea showed hyper-responsiveness to challenge testing, with 69% of these patients being diagnosed with asthma or COPD (13). Although these results have not been replicated in other dyspnoea studies, this indicates that asthma is a diagnosis that may be missed in a significant number of chronic dyspnoea patients without considering advanced respiratory function testing.
Echocardiography
Echocardiography assesses cardiac structure and function. In terms of chronic dyspnoea diagnosis, echocardiogram can identify and define conditions such as cardiac failure (HFrEF), valvular heart disease, coronary artery disease (regional wall motion abnormalities), diastolic dysfunction (HFpEF), pericardial pathology and pulmonary hypertension (8,31). Studies of chronic dyspnoea patients have suggested that echocardiography adds considerable benefit as a secondary or tertiary investigation in the diagnostic process (13,15). However, international heart failure guidelines recommend that in high risk patients with symptoms such as dyspnoea, echocardiogram should be utilised early in the diagnostic process (22,25,26). Whilst its place in the diagnostic approach to chronic dyspnoea remains under evaluation, echocardiography is nevertheless an important investigation for cardiac causes of chronic dyspnoea.
Chest computed tomography (CT)
CT of the chest can be useful in the identification of respiratory causes of dyspnoea (37,38). This modality is often used after an abnormal chest X-ray, or less commonly, if other initial testing is negative. A CT chest has a high sensitivity and specificity for diffuse parenchymal lung disease and may detect early disease such as ILD or pulmonary emphysema (13,37). There is limited data in the chronic dyspnoea setting on the diagnostic utility of CT but 69% of CTs performed in one study were abnormal, with these all leading to diagnoses such as ILD, COPD, pneumonia, pulmonary malignancy and pleural disease (13). Pulmonary oedema and cardiomegaly can also be incidentally detected on CT, although it is not a recommended modality for diagnosis. The inherent disadvantage of CT imaging is the medical radiation dose, and therefore, CT imaging requires careful patient selection by the clinician with consideration given to patient age, risk of diagnostic radiation exposure and estimated diagnostic yield. CT pulmonary angiography (CTPA) is more commonly used to detect acute pulmonary embolism; however, CTPA can also be used in suspected chronic thromboembolic pulmonary hypertension (CTEPH).
Tertiary investigations
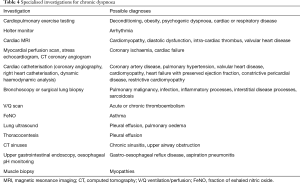
More specialised investigations may have diagnostic utility in determining the cause of chronic dyspnoea depending on the results of initial investigations and refined differential diagnoses (Table 4). Imaging modalities that may be utilised include cardiac MRI, lung ventilation/perfusion (V/Q) scans, myocardial perfusion scans, stress echocardiogram or CT coronary angiography (37,39,40). More invasive testing may include cardiac catheterisation for assessment of coronary artery disease or pulmonary pressures, muscle biopsy, bronchoscopy or surgical lung biopsy (31,41,42). Recently emerging investigations in chronic dyspnoea diagnosis include fractional exhaled nitric oxide (FeNO) testing for eosinophilic airway inflammation related to asthma, and lung ultrasound for a range of diagnoses, especially pleural effusion (17,43). There is limited data in chronic dyspnoea populations for the utility of specialised tests but it has been recommended that testing be guided by clinical judgement, clinical assessment and initial investigation results.
Full table
Cardiopulmonary exercise test (CPET)
CPET is a specialised investigation involving incremental testing of exercise capacity whilst monitored and seated on a stationary bicycle. CPET can provide valuable information on the underlying cause of dyspnoea, particularly when dyspnoea seems out of proportion to known cardiorespiratory disease (44,45). CPET measures oxygen intake, carbon dioxide elimination, minute ventilation and the maximal values of these parameters while under the stress of exercise. These measures can suggest whether the underlying diagnosis is predominantly cardiovascular or respiratory in nature (44,45). Notably, CPET can also be helpful in supporting the diagnosis of deconditioning, obesity or a psychogenic cause of dyspnoea (45). This is reflected in a study of chronic dyspnoea patients where 90% of patients with normal CPET results had a non-cardiorespiratory cause of dyspnoea diagnosed (13). In a study of CPET for unexplained chronic dyspnoea, 28% were found to have deconditioning, 32% respiratory causes, 14% cardiac causes, and 18% psychogenic causes (46). Invasive CPET is an additional option for further specialised testing to assess haemodynamics in response to exercise (47).
Diagnostic algorithms for chronic dyspnoea
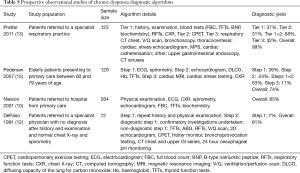
Whilst individual diagnostic investigations are useful in the diagnosis of dyspnoea, in practice, after clinical assessment, a combination of targeted investigations is often required to rule in or rule out causes of dyspnoea. The selection and prioritisation of these combinations has given rise to the development of diagnostic algorithms (Table 5).
Full table
Pratter et al. utilised an algorithm comprising three tiers of investigations with each tier resulting in approximately one third of patients being diagnosed and overall 99% of patients receiving a diagnosis for their dyspnoea (13). This algorithm was guided by clinical judgement, with tests in each tier selected by the clinicians overseeing the study, based on the results of the previous tier of testing. Whilst this method of individualised test selection by clinicians may have reduced the reproducibility of these study results, it reflects everyday clinical practice. Pedersen et al. undertook a different approach with a three-step algorithm where patients received all investigations within each step until a diagnosis was reached (15). This resulted in 73% of patients receiving a diagnosis. The recommendations from this study were for initial tests to include spirometry, ECG and chest X-ray, with echocardiogram utilised earlier in the diagnostic process (15).
Nielsen et al. conducted a study comparing the primary care assessment to a speciality clinic where patients all underwent repeat clinical assessment and a set of investigations (10). This resulted in an overall 85% diagnosis rate but with only a 39% concordance rate in diagnoses between primary care and the specialty clinic. An earlier study by DePaso et al. in 1991 evaluated a suite of investigations in patients without a diagnosis for their dyspnoea after clinical assessment, chest radiography and spirometry testing (12). Of the 72 patients participating, 81% received a final diagnosis. The sequence of testing in this study was guided by clinicians and ended once a diagnosis was made.
However, there are a number of potential limitations to these published algorithms. Firstly, the study populations of each study may not be generalisable to all patients presenting with chronic dyspnoea, especially those in primary care (Table 5). Another issue is the multifactorial nature of chronic dyspnoea, with these studies terminating their algorithms once a single diagnosis was made, which may miss additional, coexisting diagnoses. The prioritisation of investigations in some studies was unconventional; for example placing chest radiography as a third line investigation, which may limit their utility in clinical practice (15). Some studies incorporated individual clinician judgement to select testing within the algorithm, whilst others performed all investigations in all patients (10,13).
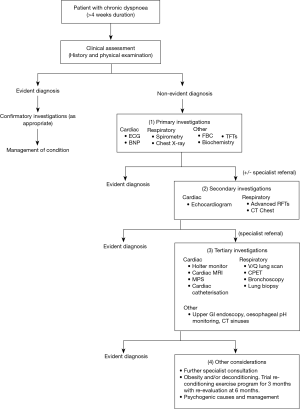
Based on the findings of these studies, we present a combined evidence-based diagnostic algorithm for chronic dyspnoea in Figure 1. This algorithm provides recommended primary, secondary and tertiary investigations in the diagnostic workup of patients with chronic dyspnoea with clinical judgement recommended at each step. This algorithm incorporates recommendations based on results from the observational studies discussed above (10,12,13,15). Importantly, future studies should test this proposed algorithm in chronic dyspnoea populations and evaluate its utility and cost-effectiveness compared to current clinical practice.
Conclusions
The diagnosis of patients with chronic dyspnoea in adults is currently challenging in both primary and tertiary care. The most common causes of chronic dyspnoea are cardiorespiratory but one third of cases have a multifactorial aetiology. This complex nature of chronic dyspnoea increases diagnostic difficulty with common comorbidities such as obesity and deconditioning often contributing to symptomatology.
This review has highlighted the inherent difficulty in chronic dyspnoea diagnosis and the diagnostic accuracy of the clinical assessment and investigations used to investigate dyspnoea. However, there are several limitations in interpretation of data. In many published studies, there was difficulty in determining the chronicity of dyspnoea studied. There was limited data available in chronic dyspnoea populations that assessed the utility of parts of the clinical assessment and investigations used in diagnosis. A number of early studies may be out-dated as diagnostic testing has advanced with greater accuracy, availability and new tests now available. Nonetheless, all of these studies of the clinical accuracy of history, examination and tests add to our knowledge about how to approach the evaluation of chronic dyspnoea.
Evidence-based diagnostic algorithms that improve both diagnostic accuracy and time to diagnosis of the cause(s) of chronic dyspnoea would be beneficial for optimising patient management. Currently, several diagnostic algorithms have been proposed to assist clinicians with chronic dyspnoea diagnostic workup. Further research is needed to evaluate these diagnostic algorithms in patients presenting to primary care to improve the diagnosis and subsequent management of patients presenting with chronic dyspnoea.
Acknowledgments
We thank the patients and staff of The Prince Charles Hospital for their involvement in our research program. We acknowledge the expertise of Chris Parker and Megan Neumann, librarians, The Prince Charles Hospital, for the literature search performed. Funding support: NHMRC Career Development Fellowship (IA Yang), NHMRC Practitioner Fellowship (KM Fong).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Karnani NG, Reisfield GM, Wilson GR. Evaluation of chronic dyspnea. Am Fam Physician 2005;71:1529-37. [PubMed]
- Berliner D, Schneider N, Welte T, et al. The Differential Diagnosis of Dyspnea. Dtsch Arztebl Int 2016;113:834-45. [PubMed]
- Currow DC, Clark K, Mitchell GK, et al. Prospectively collected characteristics of adult patients, their consultations and outcomes as they report breathlessness when presenting to general practice in Australia. PLoS One 2013;8:e74814. [Crossref] [PubMed]
- Johnson MJ, Currow DC, Booth S. Prevalence and assessment of breathlessness in the clinical setting. Expert Rev Respir Med 2014;8:151-61. [Crossref] [PubMed]
- Laviolette L, Laveneziana P. Dyspnoea: a multidimensional and multidisciplinary approach. Eur Respir J 2014;43:1750-62. [Crossref] [PubMed]
- Smith AK, Currow DC, Abernethy AP, et al. Prevalence and Outcomes of Breathlessness in Older Adults: A National Population Study. J Am Geriatr Soc 2016;64:2035-41. [Crossref] [PubMed]
- Elbehairy AF, Quint JK, Rogers J, et al. Patterns of breathlessness and associated consulting behaviour: results of an online survey. Thorax 2019;74:814-7. [Crossref] [PubMed]
- Wahls SA. Causes and evaluation of chronic dyspnea. Am Fam Physician 2012;86:173-82. [PubMed]
- Brenner S, Guder G. The patient with dyspnea. Rational diagnostic evaluation. Herz 2014;39:8-14. [Crossref] [PubMed]
- Nielsen LS, Svanegaard J, Wiggers P, et al. The yield of a diagnostic hospital dyspnoea clinic for the primary health care section. J Intern Med 2001;250:422-8. [Crossref] [PubMed]
- Huang YC, Ferry OR, McKenzie SC, et al. Diagnosis of the cause of chronic dyspnoea in primary and tertiary care: characterizing diagnostic confidence. J Thorac Dis 2018;10:3745-56. [Crossref] [PubMed]
- DePaso WJ, Winterbauer RH, Lusk JA, et al. Chronic dyspnea unexplained by history, physical examination, chest roentgenogram, and spirometry. Analysis of a seven-year experience. Chest 1991;100:1293-9. [Crossref] [PubMed]
- Pratter MR, Abouzgheib W, Akers S, et al. An algorithmic approach to chronic dyspnea. Respir Med 2011;105:1014-21. [Crossref] [PubMed]
- Pratter MR, Curley FJ, Dubois J, et al. Cause and evaluation of chronic dyspnea in a pulmonary disease clinic. Arch Intern Med 1989;149:2277-82. [Crossref] [PubMed]
- Pedersen F, Mehlsen J, Raymond I, et al. Evaluation of dyspnoea in a sample of elderly subjects recruited from general practice. Int J Clin Pract 2007;61:1481-91. [Crossref] [PubMed]
- Chang AS, Munson J, Gifford AH, et al. Prospective use of descriptors of dyspnea to diagnose common respiratory diseases. Chest 2015;148:895-902. [Crossref] [PubMed]
- Vitturi N, Soattin M, Allemand E, et al. Thoracic ultrasonography: A new method for the work-up of patients with dyspnea. J Ultrasound 2011;14:147-51. [Crossref] [PubMed]
- Davie AP, Francis CM, Caruana L, et al. Assessing diagnosis in heart failure: which features are any use? QJM 1997;90:335-9. [Crossref] [PubMed]
- Bohadana A, Izbicki G, Kraman SS. Fundamentals of lung auscultation. NEJM 2014;370:744-51. [Crossref] [PubMed]
- Oshaug K, Halvorsen PA, Melbye H. Should chest examination be reinstated in the early diagnosis of chronic obstructive pulmonary disease? Int J Chron Obstruct Pulmon Dis 2013;8:369-77. [PubMed]
- Fonseca C. Diagnosis of heart failure in primary care. Heart Fail Rev 2006;11:95-107. [Crossref] [PubMed]
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail 2016;18:891-975. [Crossref] [PubMed]
- Cottin V, Cordier JF. Velcro crackles: the key for early diagnosis of idiopathic pulmonary fibrosis? Eur Respir J 2012;40:519-21. [Crossref] [PubMed]
- Burri E, Hochholzer K, Arenja N, et al. B-type natriuretic peptide in the evaluation and management of dyspnoea in primary care. J Intern Med 2012;272:504-13. [Crossref] [PubMed]
- Atherton JJ, Sindone A, De Pasquale CG, et al. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: Guidelines for the Prevention, Detection, and Management of Heart Failure in Australia 2018. Heart Lung Circ 2018;27:1123-208. [Crossref] [PubMed]
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147-239. [Crossref] [PubMed]
- Wieshammer S, Dreyhaupt J, Basler B, et al. NT-proBNP for pulmonologists: not only a rule-out test for systolic heart failure but also a global marker of heart disease. Respiration 2009;77:370-80. [Crossref] [PubMed]
- Collerton J, Kingston A, Yousaf F, et al. Utility of NT-proBNP as a rule-out test for left ventricular dysfunction in very old people with limiting dyspnoea: the Newcastle 85+ Study. BMC Cardiovasc Disord 2014;14:128. [Crossref] [PubMed]
- Hu Z, Han Z, Huang Y, et al. Diagnostic power of the mid-regional pro-atrial natriuretic peptide for heart failure patients with dyspnea: a meta-analysis. Clin biochem 2012;45:1634-9. [Crossref] [PubMed]
- Oudejans I, Mosterd A, Zuithoff NP, et al. Applicability of current diagnostic algorithms in geriatric patients suspected of new, slow onset heart failure. Age Ageing 2012;41:309-16. [Crossref] [PubMed]
- Vogel-Claussen J, Elshafee ASM, Kirsch J, et al. ACR appropriateness criteria dyspnea: suspected cardiac origin. J Am Coll Radiol 2017;14:S127-37. [Crossref] [PubMed]
- Platz E, Hempel D, Pivetta E, et al. Echocardiographic and lung ultrasound characteristics in ambulatory patients with dyspnea or prior heart failure. Echocardiography 2014;31:133-9. [Crossref] [PubMed]
- Davenport C, Cheng EY, Kwok YT, et al. Assessing the diagnostic test accuracy of natriuretic peptides and ECG in the diagnosis of left ventricular systolic dysfunction: a systematic review and meta-analysis. Br J Gen Pract 2006;56:48-56. [PubMed]
- Davie AP, Francis CM, Love MP, et al. Value of the electrocardiogram in identifying heart failure due to left ventricular systolic dysfunction. BMJ 1996;312:222. [Crossref] [PubMed]
- Petousi N, Talbot NP, Pavord I, et al. Measuring lung function in airways diseases: current and emerging techniques. Thorax 2019;74:797-805. [Crossref] [PubMed]
- Roberts NJ, Smith SF., Partridge MR. Why is spirometry underused in the diagnosis of the breathless patient: a qualitative study. BMC Pulm Med 2011;11:37. [Crossref] [PubMed]
- Dyer DS, Mohammed TL, Kirsch J, et al. ACR appropriateness criteria chronic dyspnea: suspected pulmonary origin. J Thorac Imaging 2013;28:W64-6. [Crossref] [PubMed]
- Paech DC, Weston AR. A systematic review of the clinical effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of suspected coronary artery disease. BMC Cardiovasc Disord 2011;11:32. [Crossref] [PubMed]
- Gorantla RS, Ahmed S, Voruganti D, et al. Hyperdynamic left ventricle on radionuclide myocardial perfusion imaging (RNMPI): A marker of diastolic dysfunction in patients presenting with dyspnea on exertion. Int J Cardiol Heart Vasc 2015;9:43-7. [Crossref] [PubMed]
- Gupta A, Ghimire G, Hage FG. Guidelines in review: 2013 ACCF/AHA Guideline for the Management of Heart Failure. J Nucl Cardiol 2014;21:397-9. [Crossref] [PubMed]
- Penicka M, Bartunek J, Trakalova H, et al. Heart failure with preserved ejection fraction in outpatients with unexplained dyspnea: a pressure-volume loop analysis. J Am Coll Cardiol. 2010;55:1701-10. [Crossref] [PubMed]
- Kalluri M, Oddis CV. Pulmonary manifestations of the idiopathic inflammatory myopathies. Clin Chest Med 2010;31:501-12. [Crossref] [PubMed]
- Karrasch S, Linde K, Rucker G, et al. Accuracy of FENO for diagnosing asthma: a systematic review. Thorax 2017;72:109-16. [Crossref] [PubMed]
- Bhatt DV, Kocheril AG. Submaximal cardiopulmonary exercise testing for the evaluation of unexplained dyspnea. South Med J 2014;107:144-9. [Crossref] [PubMed]
- Toma N, Bicescu G, Enache R, et al. Cardiopulmonary exercise testing in differential diagnosis of dyspnea. Maedica 2010;5:214-8. [PubMed]
- Martinez FJ, Stanopoulos I, Acero R, et al. Graded comprehensive cardiopulmonary exercise testing in the evaluation of dyspnea unexplained by routine evaluation. Chest 1994;105:168-74. [Crossref] [PubMed]
- Biering-Sørensen T, Santos M, Rivero J, et al. Left ventricular deformation at rest predicts exercise-induced elevation in pulmonary artery wedge pressure in patients with unexplained dyspnoea. Eur J Heart Fail 2017;19:101-10. [Crossref] [PubMed]


