
Bugs at the operating theatre in infective endocarditis: one step forward, still a long way to go
Introduction
Williams et al. used data on infective endocarditis (IE) cases receiving cardiac surgery in the United States and Canada in the 2011 to 2016’ period from the Society of Thoracic Surgeons Adult Cardiac Surgery Database (STS ACSD) to investigate risk factors for 30-day mortality and major postoperative morbidity (i.e., stroke, deep sternal infection, prolonged ventilation, new onset renal failure, and/or reoperation) (1). The main novelty of the study is the inclusion of information on the causative microorganisms, whereas 2002 to 2008 data from the same database that was used to develop the STS-IE score did not include microbiological information (2). In the study at issue, 21,388 (93%) operations for left-sided and 1,698 (7%) for right-sided IE were separately analyzed. As main findings, left-sided IE perioperative mortality was not surprisingly higher than that of right-sided IE, and causative microorganisms (fungi > staphylococcal > culture negative > streptococcal) and prosthetic valve endocarditis (vs. native valve) were significantly with higher 30-day mortality in left-side IE, while for right-sided IE there were no differences in outcomes either by microorganism type or type of IE. Furthermore, length of hospital stay was significantly longer in cases caused by staphylococci and fungi than in those due to streptococci.
This is a relevant study, for two reasons mainly: the sample size and number of North American participating centers makes it a sound current picture of current practice; and yet the type of microbiological data and how it is analyzed and discussed leave still some room for improvement.
Before commenting further on Williams et al.’s study, let’s take a look at some general features of the studies dealing with the risk associated to surgically-managed IE.
Surgical risk stratification tools in IE international guidelines
“Surgery for IE carries the greatest risk of any valve surgery, and outcomes differ widely among centers and surgeons” is how the first question of the 2016 American Association for Thoracic Surgery guidelines (“Who should care for and operate on patients with IE?”) is started to be answered (3). Arguably, pursuing high-quality performance surgical risk stratification scores for IE has become the equivalent to the quest for the Holy Grail in this field of clinical research during the last decade. However, neither the latest version of the AATS (3), nor the American Heart Association (4) or the European Cardiology Society (5) guidelines included specific recommendation on the use of preoperative scores for the assessment of surgical risk in IE even though in both latter cases it is acknowledged than patients with clear indication of cardiac surgery who cannot proceed due to an unacceptable high operative risk are likely to be the subset of IE patients with worst short-term prognosis.
There is also a common agreement that such difficult clinical decisions (i.e., when to operate when the indication is evident and the risk is very high, and when to do so when the risk is moderate but the potential benefits are not so evident) should be made in a case-by-case basis in the context of a multidisciplinary team (“The Endocarditis team”) (6). Yet, the absence of compelling evidence to provide clear recommendations has hampered a general consensus on the matter so far.
Most surgical risk stratification tools for endocarditis fail to include accurate data on causative microorganisms
As shown in Table 1, there are a remarkable number of studies developing new risk scores for surgery in endocarditis, validating them with their own data, or simply analyzing the risk factors for mortality in patients with IE undergoing cardiac surgery. With the exceptions of the Costa score (7) and the most recent RISK-E score (19), data on the type of causing microorganisms is quite poor in most studies proposing new criteria or risk scores. There are mainly three reasons why this microbiological data is not good enough in most studies: first, because only information on some types of microorganisms is provided, leaving out other microorganisms with a clear prognostic impact (e.g., fungi); second, the aggrupation is not conceptually or clinically correct. Pooling together all staphylococci, when S. aureus and coagulase-negative staphylococci do not bear at all the same risk of mortality and complications, is a serious shortcoming. Furthermore, it is not clear whether enterococci are included among the “streptococci” group in those studies just providing rough categories, which is neither appropriate; and third, culture-negative endocarditis is often not contemplated. Moreover, none of the studies analyzed the impact of causative microorganisms considering at least the major antimicrobial resistance patterns, such as methicillin resistance in S. aureus (MRSA), resistance to penicillin in viridans and D group streptococci, or high-level aminoglycoside resistance and vancomycin resistance in enterococci (VRE).
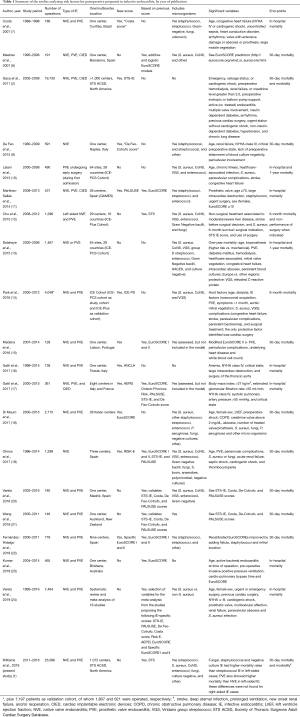
Full table
The elephant in the room: the indicated vs. performed surgery gap in endocarditis and its unclear underlying reasons
Recent large multicenter series of IE have corroborated what suspected for a long time, namely that a significant percentage of patients with indication for cardiac surgery are not operated due to a variety of reasons. Roughly half of the patients with IE have indications for surgery, of which barely two thirds are operated (22,25). Plus, this occurs when analyzing data coming from reference centers for cardiac surgery usually in urban areas, so data from smaller centers which a priori have lower rates of patients with indicated surgery due to lesser complexity are largely neglected. In the Spanish GAMES cohort, surgery was indicated in 63.9% of 1,804 patients and performed in 44.2% (26). A French survey collecting data from 303 patients with left-sided native IE found that surgery was indicated in 194 (65%) according to treating physicians and 221 (73%) according to ESC guidelines, while 139 (46%) underwent surgery. In 53% of the non-operated patients in spite of having indication for surgery, the contraindication to surgery was not reported (27). In the ICE-Plus cohort, surgery was indicated in 66.6% of cases among 1,296 patients with left-sided IE, but 25% of those with surgical indication were not operated (12). Common causes for not operating in this subgroup of patients included clinical indicators such as stroke, sepsis, and hemodynamic instability, among others. Yet, other common causes encompassed more arbitrary reasons, such as poor prognosis regardless of treatment in 30.4%, surgeon declining surgery in 22.1%, or patients refusing surgery in 12.7% (12).
Therefore, there is large variability in surgical practices across territories, as there are other determinants that are not reflected in the studies, such as whether patients might decline surgery due to economic reasons in countries without universally free healthcare systems or whether surgeons might be less likely to operate patients for reasons other than clinical or prognostic (e.g., intravenous drug users, due to ethnicity, socioeconomic status, etc.).
At least three big unsolved questions arise from these gaps: (I) how generalizable are data coming from a particular geographical setting? Or, say, can surgeons in a mid-level Italian hospital securely use scores built with data coming from large American University hospitals? Leave alone how accurate might these scores be to assess surgery in patients from rural centers in low-middle income countries; (II) how differently do surgeons respond to microbiological data when deciding therapeutic approaches? Or, do cardiac surgeons working in centers with high prevalence of invasive infections due to MRSA or VRE or culture negative IE give the same importance to the causative agent than other surgeons? and (III) do surgeons working within Endocarditis teams act differently than those who do not, including the use of risk scores? Or, do surgeons act the same with an IE case that has been diagnosed in their centers than in front a case that is referred from a smaller center? How does referral delay impact the chances of dismissing surgery due to poor clinical condition?
Databases and prospective cohorts: strengths and weaknesses
There is little doubt that the STS ACSD is a worldwide reference database for cardiac surgery. Started three decades ago, it’s a very-well coordinated initiative with high-quality data obtained through increasing completeness of queried data by compromised professionals who however are periodically audited. And more importantly for what is at stake in this commentary, by including data from more than thousand centers representing more than 90% of centers performing cardiac surgery in North America, the STS ACSD overcomes some of the frequent information biases in registries (e.g., urban-rural areas; reference-referral centers, etc.). Moreover, it provides data on postoperative morbidity relying on a multidimensional scale (see Table 1) as well as on length of stay, which are seldom included in other registries and almost never found in IE studies. It would seem of further utility to gather mortality data beyond what is reported here, at least at 6 month or better 1 year. It is presumable that a percentage of patients may have perished in recovery facilities before returning home with a potential impact in the reported mortalities.
Nationwide, population-based registries and registry-based trials have proved useful in advancing some of the answers to pivotal questions in IE. For instance, a recent study by Ahtela et al. conducted in Finland provided valuable insight on the patients’ profile and 30-day mortality of IE (28). Similarly, using multiple nationwide registries Danish investigators recently reported the rate of IE among patients with bacteremia (29).
Nevertheless, IE is a highly complex entity in both its diagnostic and clinical aspects, this limiting the validity of data coming from non-specific databases of cohorts at least in two points: accurate diagnosis is key from the epidemiological standpoint, since the identification of IE cases, undergoing surgery or not, should be done in both patients with positive and negative blood cultures and with a clear population that allows for a calculation of incidence rates. Within the pool of patients with definite and possible diagnosis of IE, which is a dynamic situation in the case of the latter, the proportion of patients eventually receiving cardiac surgery widely varies. As a consequence, the account of IE diagnoses codified from either hospital discharges (with seldom include information from autopsy records) or surgical databases in a determined timeframe is unlikely to reliably capture the complete IE picture in a large population; also, timing is a crucial variable to approach surgery, and some patients are operated in early phases or at least within the active phase of the infection, while others are operated due to residual valve regurgitation quite later, often more than a year after the first admission. The latter situation is likely to be misclassified and not be coded as IE in surgical files. In the case of the study by Williams et al., it is unclear whether all patients had a definite diagnosis of IE or some had a possible IE before surgery. Plus, pulmonic IE cases and those without an active IE were excluded.
Strictly speaking, specific IE registries and databases from prospective international or nationwide, multicenter cohorts using detailed case report forms are a more reliable source. In spite of their limitations, which also include epidemiological representativeness, as well as referral bias including cardiac surgery rates, and in some cases lack of relevant variables such as relapses, prospective cohorts provide detailed and trustful data tailored to be interpreted in the complex context of IE. Some examples of this are the International Collaboration of Endocarditis, the EURO-ENDO including 156 centers from 40 European countries, the ID-RI study encompassing 13 also European countries, the VIRSTA/AEPEI cohort in France, the GAMES cohort in Spain, or the East Danish Database on Endocarditis.
Unfortunately, one size does not fit all in the case of surgery for IE. While surgical databases usually lack important information such as microbiology or antibiotic treatment, specific prospective IE cohorts frequently lack key surgical data (e.g., detailed information on the type of surgery, post-operative morbidity, reoperations during the long-term follow-up, etc.). Both types of sources complement each other, but none is sufficient by itself, like a maladjusted set of Chinese of boxes. Williams and colleagues’ study, in other words the inclusion of microbiological data in the STS ACDS, is a meritorious attempt to fix it by building a bridge. However, one of the major limitations is failing to report the acuity in which surgery (elective, urgent or emergent) has been performed as this provides a real picture of the patient population allowing appropriate understanding and comparison of data to other cohorts.
STS ACDS and the bugs: findings and unresolved issues
Although Williams et al.’s study goes far beyond some other studies (Table 1) at providing microbiological data, i.e., it is not limited to staphylococci, streptococci and other, there are at least five shortcomings regarding variables and analysis related to causative microorganisms worth mentioning. First, in spite of showing the outcomes separately for S. aureus and coagulase-negative staphylococci (CoNS) in the tables, the abstract, discussion and conclusions are based on a jointly consideration of both, also mixing up how microbiological data are provided for native and prosthetic valve IE when separated by left or right-sided involvement. This is of utmost relevance, since the epidemiological trends in different geographical settings, the type of IE typically caused by either S. aureus or CoNS, their aggressiveness and potential antibiotic options are neatly different (30). For instance, it is not surprise that that root replacement was found to be more frequent among IE cases due to S. aureus than in those caused by CoNS, since the tissue destruction, especially in native valve endocarditis is much greater in the case of the former (although there are exception of high virulence in CoNS IE, such as those caused by S. lugdunensis and S. capitis). Secondly, it is not clear whether microbiological information originated from valve samples obtained during surgery was incorporated, and thus the doubt remains on whether the 11% of culture-negative endocarditis is “blood culture negative” or “all culture” negative IE. Thirdly, the appraisal of the variability of the length of stay in relation with the causative microorganism would have been much more comprehensive should any data on the antimicrobial treatment and the availability of outpatient parenteral antibiotic treatment have been provided. Fourth, the type of acquisition (community or healthcare-associated, including nosocomial and non-nosocomial) is missing and would have been a powerful tool to interpret the microbiological findings. Finally, the percentage of reoperations (included in the composite major morbidity index) corresponding to very-early onset PVE or relapses is not disclosed.
From an etiologic perspective, the epidemiological findings of the William et al.’s study are certainly surprising, since most studies performed during the last decade showed an increase in staphylococcal IE and enterococcal IE and a decrease of streptococcal IE in industrialized countries, including North America (31). A 15% of left-sided IE and 5% of right-sided IE caused by enterococci are consistent figures, while the fact that streptococci appear to be the first causative microorganism of operated left-sided IE in North America requires further reflection. In our opinion, it might be explained by various reasons. It is still unclear based on the current literature whether the changes in 2007 AHA IE prophylaxis guidelines have entailed an increase in streptococcal IE (32,33). Another plausible explanation for this phenomenon is that most studies reporting data on operated patients come from large referral centers where the percentage of complicated staphylococcal cases is likely higher than in small-medium centers without cardiac surgery, while the seldom reported epidemiology in surgical centers from non-urban areas is quite different. This would imply that the percentage of healthcare-associated IE in North America is far lower than reported to date (34). The same could be said of the low percentage (1%) of fungal IE found in left-sided IE.
Regarding staphylococci, the unfortunate implications of not including the percentage of MRSA are twofold: it would have allowed contrasting the findings with recent reports pointing to a decrease in methicillin-resistant and an increase in methicillin-susceptible S. aureus invasive infections in the U.S. (35,36), and methicillin-resistance should be considered in case the STS-IE score is updated with microbiological data.
Also regrettable is the lack of information regarding the use of intravenous drugs in the sample, since it would have enabled the authors to link their findings with the increase in IE among intravenous drug users (IDUs) in the context of the current opioid crisis. Even though the study period (2011 to 2016) would have not captured the peak of the epidemics, a recent nationwide study has shown a significant increase in IDUs-IE admissions between 2010 and 2015 (37). In any case, comparing the rates of surgery and recidivism among IDUs with those of the nineties, especially in patients with HIV infection, is a burgeoning field of interest. Williams and colleagues might have shed light in some complicated issues, such as the outcomes of operated IE with involvement of both left and right valves in IDUs, since most than 10% of the left-sided IE group, 2,399 patients, had both left and right involvement.
Finally, the way the information regarding the multivariable analysis is somehow confusing. No variables other than the causative microorganisms are shown in the results of the multivariable analysis [left sided IE in Table 2 and right-side IE in Table 3 in (1)] in the main article except for the comparison between native and prosthetic valve IE [Table 4 in (1)]. The other factors included in the multivariable analysis are displayed apart (in the Supplemental material). This hampers how readership might or not obtain a comprehensive insight on the actual weight of causative microorganisms on mortality and major postoperative morbidity. For example, both outcomes were significantly worst for prosthetic than native valve IE in left-sided cases, but how much did the fact that prior coronary artery bypass graft surgery was 22.8% in the former and 3.3% in the latter, or mean age being 62 and 55 years, and peripheral vascular disease 13.5% and 9.7% respectively impact outcomes? In addition, no information is given on preoperative intra-aortic balloon pump, multiple valve procedure, and NYHA classification.
The way forward
In summary, Williams and colleagues are to be complimented for pioneering the inclusion of relevant microbiological data in the STS ACSD, which surely will lead to more refined insights on the impact of causative microorganisms in the near future and, more importantly, will set a standard for the upcoming surgical risk scores in IE (maybe an STS-MIE score with an “M” for microbiology? Or perhaps an “E” for enhanced?).
Meanwhile, there are important gaps still pending of resolution after this study highlighting the importance of understanding the intricacies of the etiology in IE, which cannot be addressed in isolation. However, the gaps do not only concern high-quality surgical databases such as STS ACSD: there is an imperative need for prospective international cohort IE studies that meticulously collect relevant data on cardiac surgery, and fulfill the dire necessity to evaluate how the surgical management of IE within well-oiled Endocarditis teams impacts on outcomes, and how the surgical decisions within these teams are conditioned by microbiological insights.
Acknowledgments
JM Miró received a personal 80:20 research grant from the Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, Spain, during 2017 to 2019.
Footnote
Conflicts of Interest: JM Miró has received consulting honoraria and/or research grants from AbbVie, Bristol-Myers Squibb, Contrafect, Cubist, Genentech, Jansen, Medtronic, Novartis, Gilead Sciences, and ViiV, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Williams JB, Shah AA, Zhang S, et al. Impact of microbiological organism type on surgically managed endocarditis. Ann Thorac Surg 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Gaca JG, Sheng S, Daneshmand MA, et al. Outcomes for endocarditis surgery in North America: a simplified risk scoring system. J Thorac Cardiovasc Surg 2011;141:98-106.e1-2.
- AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee Chairs, Pettersson GB, Coselli JS, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: Surgical treatment of infective endocarditis: Executive summary. J Thorac Cardiovasc Surg 2017;153:1241-58.e29. [Crossref] [PubMed]
- Baddour LM, Wilson WR, Bayer AS, et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2015;132:1435-86. [Crossref] [PubMed]
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36:3075-128. [Crossref] [PubMed]
- Chambers J, Sandoe J, Ray S, et al. The infective endocarditis team: recommendations from an international working group. Heart 2014;100:524-7. [Crossref] [PubMed]
- Costa MA, Wollmann DR Jr, Campos AC, et al. Risk index for death by infective endocarditis: a multivariate logistic model. Rev Bras Cir Cardiovasc 2007;22:192-200. [PubMed]
- Mestres CA, Castro MA, Bernabeu E, et al. Preoperative risk stratification in infective endocarditis. Does the EuroSCORE model work? Preliminary results. Eur J Cardiothorac Surg 2007;32:281-5. [Crossref] [PubMed]
- De Feo M, Cotrufo M, Carozza A, et al. The need for a specific risk prediction system in native valve infective endocarditis surgery. ScientificWorldJournal 2012;2012:307571. [Crossref] [PubMed]
- Lalani T, Chu VH, Park LP, et al. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med 2013;173:1495-504. [Crossref] [PubMed]
- Martínez-Sellés M, Muñoz P, Arnáiz A, et al. Valve surgery in active infective endocarditis: a simple score to predict in-hospital prognosis. Int J Cardiol 2014;175:133-7. [Crossref] [PubMed]
- Chu VH, Park LP, Athan E, et al. Association between surgical indications, operative risk, and clinical outcome in infective endocarditis: a prospective study from the International Collaboration on Endocarditis. Circulation 2015;131:131-40. [Crossref] [PubMed]
- Delahaye F, Chu VH, Altclas J, et al. One-year outcome following biological or mechanical valve replacement for infective endocarditis. Int J Cardiol 2015;178:117-23. [Crossref] [PubMed]
- Park LP, Chu VH, Peterson G, et al. Validated Risk Score for Predicting 6-Month Mortality in Infective Endocarditis. J Am Heart Assoc 2016;5:e003016. [Crossref] [PubMed]
- Madeira S, Rodrigues R, Tralhão A, et al. Assessment of perioperative mortality risk in patients with infective endocarditis undergoing cardiac surgery: performance of the EuroSCORE I and II logistic models. Interact Cardiovasc Thorac Surg 2016;22:141-8. [Crossref] [PubMed]
- Gatti G, Benussi B, Gripshi F, et al. A risk factor analysis for in-hospital mortality after surgery for infective endocarditis and a proposal of a new predictive scoring system. Infection 2017;45:413-23. [Crossref] [PubMed]
- Gatti G, Perrotti A, Obadia JF, et al. Simple Scoring System to Predict In-Hospital Mortality After Surgery for Infective Endocarditis. J Am Heart Assoc 2017. [Crossref] [PubMed]
- Di Mauro M, Dato GMA, Barili F, et al. A predictive model for early mortality after surgical treatment of heart valve or prosthesis infective endocarditis. The EuroSCORE. Int J Cardiol 2017;241:97-102. [Crossref] [PubMed]
- Olmos C, Vilacosta I, Habib G, et al. Risk score for cardiac surgery in active left-sided infective endocarditis. Heart 2017;103:1435-42. [Crossref] [PubMed]
- Varela L, López-Menéndez J, Redondo A, et al. Mortality risk prediction in infective endocarditis surgery: reliability analysis of specific scores. Eur J Cardiothorac Surg 2018;53:1049-54. [Crossref] [PubMed]
- Wang TKM, Pemberton J. Performance of Endocarditis-Specific Risk Scores in Surgery for Infective Endocarditis. Thorac Cardiovasc Surg 2018;66:333-5. [Crossref] [PubMed]
- Fernández-Hidalgo N, Ferreria-González I, Marsal JR, et al. A pragmatic approach for mortality prediction after surgery in infective endocarditis: optimizing and refining EuroSCORE. Clin Microbiol Infect 2018;24:1102.e7-1102.e15. [Crossref] [PubMed]
- Kumar A, Anstey C, Tesar P, et al. Risk factors for mortality in patients undergoing cardiothoracic surgery for infective endocarditis. Ann Thorac Surg 2019;108:1101-6. [Crossref] [PubMed]
- Varela Barca L, Navas Elorza E, Fernéndez-Hidalgo N, et al. Prognostic factors of mortality after surgery in infective endocarditis: systematic review and meta-analysis. Infection 2019. [Epub ahead of print].
- Cahill TJ, Baddour LM, Habib G, et al. Challenges in Infective Endocarditis. J Am Coll Cardiol 2017;69:325-44. [Crossref] [PubMed]
- Muñoz P, Kestler M, De Alarcon A, et al. Current Epidemiology and Outcome of Infective Endocarditis: A Multicenter, Prospective, Cohort Study. Medicine (Baltimore) 2015;94:e1816. [Crossref] [PubMed]
- Iung B, Doco-Lecompte T, Chocron S, et al. Cardiac surgery during the acute phase of infective endocarditis: discrepancies between European Society of Cardiology guidelines and practices. Eur Heart J 2016;37:840-8. [Crossref] [PubMed]
- Ahtela E, Oksi J, Porela P, et al. Trends in occurrence and 30-day mortality of infective endocarditis in adults: population-based registry study in Finland. BMJ Open 2019;9:e026811. [Crossref] [PubMed]
- Østergaard L, Bruun NE, Voldstedlund M, et al. Prevalence of infective endocarditis in patients with positive blood cultures: a Danish nationwide study. Eur Heart J 2019;40:3237-44. [Crossref] [PubMed]
- Wang A, Gaca JG, Chu VH. Management Considerations in Infective Endocarditis: A Review. JAMA 2018;320:72-83. [Crossref] [PubMed]
- Slipczuk L, Codolosa JN, Davila CD, et al. Infective endocarditis epidemiology over five decades: a systematic review. PLoS One 2013;8:e82665. [Crossref] [PubMed]
- Thornhill MH, Gibson TB, Cutler E, et al. Antibiotic Prophylaxis and Incidence of Endocarditis Before and After the 2007 AHA Recommendations. J Am Coll Cardiol 2018;72:2443-54. [Crossref] [PubMed]
- Pant S, Patel NJ, Deshmukh A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol 2015;65:2070-6. [Crossref] [PubMed]
- Benito N, Miró JM, de Lazzari E, et al. Health care-associated native valve endocarditis: importance of non-nosocomial acquisition. Ann Intern Med 2009;150:586-94. [Crossref] [PubMed]
- Austin ED, Sullivan SS, Macesic N, et al. Reduced mortality of Staphylococcus aureus bacteremia in a retrospective cohort study of 2139 patients: 2007 - 2015. Clin Infect Dis 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Acree ME, Morgan E, David MZ. S. aureus Infections in Chicago, 2006-2014: Increase in CA MSSA and Decrease in MRSA Incidence. Infect Control Hosp Epidemiol 2017;38:1226-34. [Crossref] [PubMed]
- Rudasill SE, Sanaiha Y, Mardock AL, et al. Clinical Outcomes of Infective Endocarditis in Injection Drug Users. J Am Coll Cardiol 2019;73:559-70. [Crossref] [PubMed]


