
Natural evolvement of lung tumors induced by N-ethyl-N-nitrosourea (ENU) and the impact of a high sucrose-high fat diet on tumor evolvement assessed by tumor histology in inbred BALB/c and C57BL/6J mice
Introduction
N-ethyl-N-nitrosourea (ENU), an alkylating agent, was widely used to study tumorigenesis by murine models from 50s to 80s last century (1-3) [reviewed by Shimkin and Stoner (4)]. Single intraperitoneal (i.p.) injection of ENU at a moderate dose in gravid mice at late stages of gestation transplacentally induced tumors mainly in the lung of the offspring (1-5). The authors adapted the method and successfully established a model in an inbred BALB/c strain (6). Approximately 1/3 of ENU-induced lung tumors in our model naturally evolved to adenocarcinomas at age of 32 weeks. There were no significant differences in the frequency of induction of lung tumors and of malignant transformation between genders though some differences in the morphology of lung adenocarcinomas were noted (6).
The association between high fat diets and cancer development had been investigated for several decades [reviewed by Font-Burgada et al. (7)]. Epidemiological investigations observed that foods rich in fat were associated with increased risk of cancer development (8-11), including lung cancer (12,13). Preclinical studies by various models demonstrated that high-fat or high sucrose-high fat (HSHF) diet promoted the development of cancers (14-16); and the underlying mechanisms were explored (16-18).
The model established by our research group was used to investigate the impact of a HSHF diet on lung tumorigenesis parallel in two inbred strains, BALB/c (B/c) and C57BL/6J (B6J). The entire lungs of ENU- or buffer-treated mice were harvested at age of 24 weeks and processed by a standard procedure of paraffin-embedded blocks. Those lungs were cut in serial sections but step sections of every 5th (step sections in 5) were saved and stained with hematoxylin and eosin (HE). The present study mainly reported findings in the histology of ENU-induced lung tumors and the impact of the HSHF diet on the progression of those tumors.
Methods
Animals and foods
B6J and B/c inbred mice (SPF grade), 4 to 6 weeks old, were purchased from animal facility center of Guangzhou University of Chinese Medicine (animal certificates: No. 44005800000798 and No. 44005800001289). After 1-week quarantine they were bred in the laboratory of our animal facility under the control of temperature (20 to 26 Celsius), humidity (40% to 70%) and 12 h-12 h light-dark cycles. The sterile water was supplied. Food and water were taken ad libitum. Mice of 2 strains were bred in the same laboratory.
Both the standard and the HSHF diet were provided by Guangdong Animal Center for Medical Research. They were sterilized by 60Co. The main ingredients in those diets were listed in Table 1. The calories produced by the standard and the HSHF diets were 3.4 kcal/g and 4.4 kcal/g, respectively. The procedures and conduction of the experiments were reviewed and approved by Animal Welfare Committee of Guangzhou Medical University (certificate of animal study: No. 00098221). All technicians and clinicians were certified to do animal work in the facility.
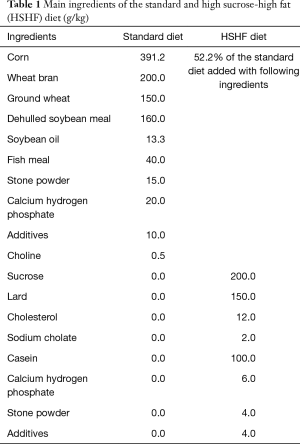
Full table
Mating and calculation of gestation days
When females were at least 10 weeks old and their body weights reached 19 to 20 g for B6J and 21 to 22 g for B/c, mating was carried out between 1 male and 1 female. Each female mouse was taken into the cage of individually housed male from 5pm the previous day to about 8am the next morning. After overnight mating, males were taken out of the cages. Males rested for one week prior to another round of mating. Gestation was determined 2 weeks post mating. Those not in gravid would go into another round of mating. Most of the mating were managed to go between brothers and sisters. The mating of the 2 strains was conducted in the same laboratory. Gestation days were arbitrarily recorded as day 0 on the day of mating, day 0.5 at the time of males taken out of the mating cages, and so forth.
Injection of ENU or buffer
ENU (Sigma-Aldrich, N3385-1G, purchased from Guangzhou life science business area) was divided into 5 to 20 mg aliquots in sterile glass tubes upon arrival and stored at −20 °C. It was prepared before the injection at a concentration of 4 mg/mL by Na2HPO4/citric acid buffer (pH 6.0) that was filtered by 0.25-µm filter. The buffer was prepared in advance and stored at 4 °C.
The gravid mice of both strains on the 17th day of gestation received an intraperitoneal (i.p.) injection of either ENU (experimental group) at 40 mg/kg body weight or vehicle buffer (control group) that was equivalent to ENU injection by body weight. No mice died after the injection in both groups. ENU- or buffer-injected females were individually housed and allowed to deliver as usual. They were switched to a new cage with their litters 24 hours post delivery. The litters were weaned on day 25. The mothers were euthanized after weaning.
Feeding with the standard or HSHF diet
The offspring were randomly separated by gender upon weaning. Both ENU- and buffer-treated offspring in each strain were further divided into 2 subgroups by each gender. One subgroup was put on the standard diet and the other was on the HSHF diet from weaning to week 24 when they were sacrificed.
Sample collection, processing and histological examination
Mice were fasted of food for 4 to 5 hours before necropsy. Each lung was inflated and fixed with a phosphate-buffered formalin (commercially purchased, Guangzhou, China). After 24 to 48 hours’ fixation, the trachea was cut out. All collected lungs went through a standard procedure for paraffin-embedded blocks in an automatic tissue processor (Leica, Shanghai, China).
The number of mice that their lungs were examined in each subgroup was listed in Table 2. The paraffin-embedded blocks were randomly selected for histological examination. Whole blocks of the lungs were cut in serial 5 µm sections but only every 5th (one 5 µm section per 25 µm intervals, step sections in 5) were saved for staining by hematoxylin and eosin (HE; Leica, Shanghai, China). Each block yielded 60 to 70 step sections. The histology of lung tumors was assessed by a surgical pathologist (M.H.) in our research group.
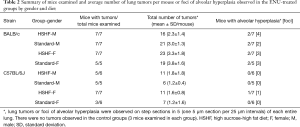
Full table
Results
Histological findings in the development of lung tumors
The earliest changes in the lung caused by ENU manifested as that the alveolar walls were still discernible but lining cells became hyperplastic (Figure 1A) and gradually filled the space of an alveolus to form a spot of solid area (Figure 1B). A lung tumor was developed when the air space of several adjacent aveoli were filled by hyperplastic alveolar cells to form a solid nodule. The alveolar hyperplasia was regarded as a lesion prior to tumor formation but not counted as a tumor in our bioassays. The lesion was not easily seen in the B6J strain (Table 2). Lung tumors derived from hyperplastic alveolar cells were located close to the pleura and some of them were in touch with it.
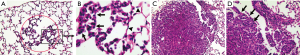
The hyperplasia of bronchiolar epithelial cells was not observed. Some tumors grew around terminal bronchioles and their extending breathing alveolar duct (Figure 1C). The mode of tumor growth (Figure 1D) implied that those tumors were probably developed either from the epithelial cells lining the bronchiole at the very end or from alveolar cells adjacent to the breathing alveolar duct. This group of tumors was still located in the periphery of the lung but farther from the pleura. Only a small proportion of tumors scattered in the parenchyma of the lung but without any signs to be in touch with a bronchiole on step sections. Those tumors were possibly developed from alveolar cells as well.
All mice examined were observed to get lung tumors in the B/c strain and the average number of lung tumors per mouse was similar in each subgroup regardless of gender and diet (Table 2). Whereas, 4 out of 24 mice examined in the B6J strain did not get lung tumors [Table 2; 1 mouse in the male group on the HSHF diet (5/6) and 3 mice in the female group on the standard diet (3/6)]. But the average number of lung tumors per mouse remained similar in each subgroup, also regardless of gender and diet (Table 2). There seemed to be more tumors per mouse in the B/c strain than that in the B6J strain but the data would not be further analyzed in the present study.
None of 24 mice examined from all buffer-treated groups on both diets were observed to develop foci of alveolar hyperplasia or lung tumors.
Histological findings in the natural evolvement of lung tumors
Lung tumors manifested a variety of histological changes. Some tumors were composed of cells that looked uniform in shape and size with small centered and bland nuclei (Figure 2A,B); and were usually solid without any obvious acini or papillae. Whereas, some others looked complex as cellularity was partly increased (Figure 2C,D) and cells varied in sizes (Figure 2C,D,E,F,G,H and Figure 3). More importantly, a small proportion of tumor cells with nuclear dysplasia at various degrees appeared in the preexisted cells and overlapped with those cells, which made a particular tumor look as if it was composed of layers of tumor cells (Figure 2C and Figure 3C,D,E,F). At the same time, the acinar or papillary or mixed histological structures developed in a solid tumor (Figure 2E,G and Figure 3A,C,E).
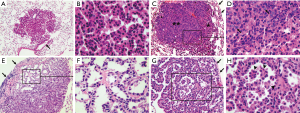
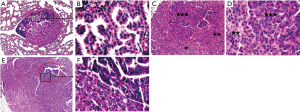
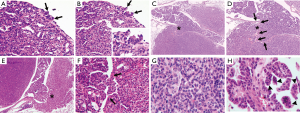
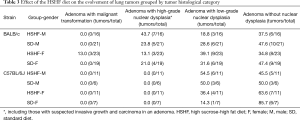
The morphological variations in lung tumors outlined above demonstrated that tumor cells naturally evolved not only with time, but also with different paces, which resulted in that lung tumors were at different developmental stages at the checkpoint of week 24 (Figures 2,3). Tumors without nuclear dysplasia (Figure 2A,B) or with mild to moderate degree of nuclear dysplasia (Figure 2C,E,G) were at early stages; and could be pathologically defined as adenomas or adenomas with low-grade dysplasia. Those with nuclear dysplasia at severe degree (Figure 3A,C,E) were at late stages and could be defined as adenoma with high-grade dysplasia; and a few of them possibly reached a status of “carcinoma in an adenoma” in a cytological view (Figure 3E,F) or suspected focal invasive growth (Figure 4A,B). Thus, lung tumors could be classified into different categories according to their histological presentation; and the comparison in percentages of total tumors observed could be made between the strains. Clearly, there were some percentages of lung tumors in the category of late stage in the B/c strain but not in the B6J strain (Table 3) on the standard diet.
Full table
Histological findings in the impact of the HSHF diet on lung tumors
The HSHF diet accelerated the rate of natural evolvement of lung tumors in both strains based on 2 facts. One was that there was a trend that a proportion of tumors shifted from a lower stage to a higher stage in both strains on the HSHF diet (Table 3). Another was that 3 tumors got spots of malignant transformation in 2 females of the B/c strain determined by invasive growth (Figure 4C,D,E,F). Those tumors could be defined as adenocarcinomas. In one of the tumors, cancer cells in the bronchiolar lumen poked the wall of the bronchiole and invaded the same tumor on the other side (Figure 4E,F). The tumor cells with high-grade nuclear dysplasia (Figure 4G) looked similar to cancer cells in the lumen of the bronchiole (Figure 4H). Therefore, the invasive growth was a more reliable sign to define malignancy.
The impact of the HSHF diet on the evolvement of lung tumors seemed to be influenced by gender, stronger at earlier stages in males in both strains. As tumors reached the late stage (cells with high-grade nuclear dysplasia), the diet sped up the tumor progression to become adenocarcinoma only in females in the B/c strain (Table 3).
Another unexpected finding was that a cluster of tumor cells were found to become “fatty” with wide and clear or foamy cytoplasm (Figure 5) in both strains in ENU-treated groups on the HSHF diet. A few of signet-ring shaped cells were scattered here and there but mostly among cells with clear or foamy cytoplasm (Figure 5B,C). The frequency was 8 tumors from 7 mice out of total 61 tumors from 27 mice in both strains on the HSHF diet. The tumors with such cytological features could be adenomas (Figure 5A,B,C) or adenomas with dysplasia at various degrees or tumors with invasive growths (Figure 5D,E,F). None of the tumors on the standard diet was observed to have such changes in the present and previous (6) studies.
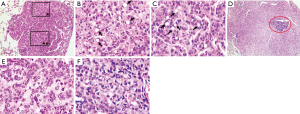
Foamy and signet-ring cells were observed in lung tumors on the high sucrose-high fat (HSHF) diet. (A) Clusters of foamy and signet-ring cells could be seen in a tumor with nuclear dysplasia at a mild to moderate degree (original 100×). (B) The squared area* in (A) was magnified. Tumor cells got wide and foamy cytoplasm. A few of signet ring cells (arrows) were scattered among them (original 400×). (C) The squared area** in (A) was magnified. A few of signet ring cells (arrows) were found though other cells did not show any foamy cytoplasm (original 400×). (D) One step section of the same tumor shown in the Figure 3E displayed that the tumor seemed to have some light areas mingled with dark ones (original 40×). The area in red oval circle was the marker of the tumor. (E,F) Two light areas were magnified to show that most of cells had wide, light and foamy cytoplasm; and a few of signet ring cells could be seen in F (E and F, original 400×). Those cells got nuclear dysplasia at a severe degree. All figures presented here were taken from hematoxylin and eosin-stained slides.
Discussion
The primary purpose of our study was to explore the possible effects of the HSHF diet on the development of lung tumors induced by ENU and to see if the effects would be differential between the 2 strains. From our previous experiences (6), the checkpoint was chosen at week 24 for the reason that this was the critical time point to observe malignant transformation if the diet would have any positive impact on tumor development and progression. Step sections in 10 (one 5 µm section per 50 µm interval) was reported equivalent to that by serial sections in assessing the incidence, histological type, size of all proliferative processes (19) and number of tumors (20) in the entire lung. The authors got similar experiences by step sections in 5 (one 5 µm section per 25 µm interval) in the present study (Tables 2,3). Although numbers of tumors varied from mouse to mouse (Table 2), tumor pathology was fairly informative and comparable from mouse to mouse and between the 2 strains.
By step sections in 5, the authors could define for the first time that the low- or high-grade nuclear dysplasia was an entity of lung tumors induced by ENU; and the entity showed that a cluster of tumor cells in a particular tumor were evolving step-by-step towards malignancy (Figures 3,4). Because of those constantly evolving cells, a proportion of lung tumors were doomed to become malignant at a certain point. The appearance of high-grade dysplasia in tumor cells accompanied with histological restructuring indicated tumor progression. Fibrotic reaction and inflammatory infiltration in the pleura (Figure 2E) in tumors in touch with it reflected that the tumors were at an advanced stage. Back in 1971, Kimura (21) did conclude from his elegant histological and transplanted studies that different histological patterns of tumor structure represented a status of tumor progression. In fact, the evolvement of lung tumors was outlined by the spectrum of morphological changes (from focal hyperplasia of alveolar cells, adenomas in solid, papillary or mixed forms to adenocarcinomas) but was neither considered nor signified by other investigators (20-23). Tumor structures were more emphasized than the cytology of tumor cells.
Thus, tumor histology was instrumental to discriminate the nature of a tumor, probably more precise than tumor number or size. The inflation of the lungs by the buffered fixative and proper step sections were essential to get precise histological assessment. Based on the tumor histology, it was possible for us to compare percentages of lung tumors in different categories and effects of the HSHF diet on the tumor development and progression between genders and strains.
In the present study, the HSHF diet was given on the day of weaning when the ENU administration had completed for 26 to 27 days. Since the number of lung tumors was not affected by the diet in both strains while compared with the groups on the standard diet (Table 2), it indicated that the diet had little impact on tumor development. The main effects of the diet were to accelerate evolving of tumor cells (Table 3). In the B/c strain such effects were more prominent and resulted in lung adenocarcinoma developed (3/23 lung tumors from 2/7 female mice examined) as compared with those on the standard diet (0/19 tumors from 7 female mice examined). In reality, the effects of similar HSHF diets might be very complex, depending on the time of administration. Combining or synergic effects with the carcinogenic agents might be present if given simultaneously (15,16).
Tumor cells with wide foamy or clear or signet-ring cytoplasm (Figure 5) appeared in 8 tumors from 7 mice out of total 61 tumors from 27 mice of both strains on the HSHF diet. Since those cells appeared not only in the tumors with the invasive growth (Figure 5D,E,F) but also in adenomas with nuclear dysplasia at various degrees (Figure 5A,B,C), the authors assumed that the occurrence of such cells might be an early event that was separated from tumor evolvement. In reviewing of research papers, the authors learned that in a few benign tumors, foamy and signet ring cells were also observed (24-28) but only few were positively stained with mucin (27). At the moment we could not offer any explanations but proposed a hypothesis that those clusters of foamy cells might acquire ability, induced by a long term of administration of the HSHF diet, to actively store lipids or glycogen or mixture of both in their cytoplasm like liver cells. The significance was unknown. Certainly, additional study on our hypothesis was assured.
As for our proposal that lung tumors developed at different times, the authors would like to refer the finding that the number of lung tumor increased with time and plateaued at a certain point in the Swiss-Webster and C3H mice reported by other investigators (29,30). Since fetal lung development and the proliferation of alveolar cells affected the induction of lung tumors (2,3,31), different gestational durations among strains might contribute to the timing of alveolar development and proliferating index of alveolar cells, therefore, to the efficacy of neoplastic transformation of alveolar cells by ENU. The authors had observed that gestational durations was 0.3 days significantly longer in the B6J stain than in the B/c strain (32).
Conclusions
The spectrum of histological changes of lung tumors at the observing time point, together with the appearance of low- or high-grade dysplasia, determined lung tumors developed at different times and naturally evolved at different paces. In fact, those tumors were at different developmental stages. The HSHF diet accelerated the course of natural evolvement and promoted adenocarcinoma developed in female mice of the B/c strain. The present study laid a foundation for further analysis of the effects expressed by the HSHF diet. On the whole, lung tumors in the B6J strain were less in number and less advanced in terms of developmental stages than those in the B/c strain (Tables 2,3); but a full comparison between the 2 strains was beyond the scope of the present study and would assure a further investigation.
Acknowledgments
The authors would like to express their sincere thanks to technicians in our animal facility for their assistance in animal care, Jinhui Feng for his generous assistance in tissue processing and slides preparation, and technicians in the Department of Pathology at affiliated Cancer Hospital & Institute of Guangzhou medical College for their technical support.
Funding: This work was supported by the Science and Technology Foundation of Guangdong Province (2013B060300023) to Lijun Dai.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The procedures and conduction of the experiments were reviewed and approved by Animal Welfare Committee of Guangzhou Medical University (certificate of animal study: No. 00098221). All technicians and clinicians were certified to do animal work in the facility.
References
- Searle CE, Jones EL. The multipotential carcinogenic action of N-ethyl-N-nitrosourea administered neonatally to mice. Br J Cancer 1976;33:612-25. [Crossref] [PubMed]
- Kauffman SL. Susceptibility of fetal lung to transplacental 1-ethyl-1-nitrosourea: its relation to epithelial proliferation. J Natl Cancer Inst 1976;57:821-5. [Crossref] [PubMed]
- Diwan BA, Meier H. Strain- and age-dependent transplacental carcinogenesis by 1-ethyl-1-nitrosourea in inbred strains of mice. Cancer Res 1974;34:764-70. [PubMed]
- Shimkin MB, Stoner GD. Lung tumors in mice: application to carcinogenesis bioassay. Adv Cancer Res 1975;21:1-58. [Crossref] [PubMed]
- Vesselinovitch SD, Rao KV, Mihailovich N. Neoplastic response of mouse tissues during perinatal age periods and its significance in chemical carcinogenesis. Natl Cancer Inst Monogr 1979.239-50. [PubMed]
- Hou M, Dai LJ, Tan XJ, et al. Establishment and pathological characterization of a mouse model of lung adenocarcinoma. Acta Lab Anim Sci Sin 2012;20:75-9.
- Font-Burgada J, Sun B, Karin M. Obesity and cancer: the oil that feeds the flame. Cell Metab 2016;23:48-62. [Crossref] [PubMed]
- Gordon-Dseagu VLZ, Thompson FE, Subar AF, et al. A cohort study of adolescent and midlife diet and pancreatic cancer risk in the NIH-AARP diet and health study. Am J Epidemiol 2017;186:305-17. [Crossref] [PubMed]
- Koh WP, Dan YY, Goh GB, et al. Dietary fatty acids and risk of hepatocellular carcinoma in the Singapore Chinese health study. Liver Int 2016;36:893-901. [Crossref] [PubMed]
- Sieri S, Chiodini P, Agnoli C, et al. Dietary fat intake and development of specific breast cancer subtypes. J Natl Cancer Inst 2014;106:dju068. [Crossref] [PubMed]
- Ruder EH, Thiebaut AC, Thompson FE, et al. Adolescent and mid-life diet: risk of colorectal cancer in the NIH-AARP Diet and Health Study. Am J Clin Nutr 2011;94:1607-19. [Crossref] [PubMed]
- Yang JJ, Yu D, Takata Y, et al. Dietary fat intake and lung cancer risk: a pooled analysis. J Clin Oncol 2017;35:3055-64. [Crossref] [PubMed]
- Wu Y, Zheng W, Sellers TA, et al. Dietary cholesterol, fat, and lung cancer incidence among older women: the Iowa Women's Health Study (United States). Cancer Causes Control 1994;5:395-400. [Crossref] [PubMed]
- Tang ZC, Shivapurkar N, Frost A, et al. The effect of dietary fat on the promotion of mammary and colon cancer in a dual-organ rat carcinogenesis model. Nutr Cancer 1996;25:151-9. [Crossref] [PubMed]
- Hoffmann D, Rivenson A, Abbi R, et al. A study of tobacco carcinogenesis: effect of the fat content of the diet on the carcinogenic activity of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in F344 rats. Cancer Res 1993;53:2758-61. [PubMed]
- Sundaram S, Yan L. High-fat Diet Enhances Mammary Tumorigenesis and Pulmonary Metastasis and Alters Inflammatory and Angiogenic Profiles in MMTV-PyMT Mice. Anticancer Res 2016;36:6279-87. [Crossref] [PubMed]
- Dermadi D, Valo S, Ollila S, et al. Western Diet Deregulates Bile Acid Homeostasis, Cell Proliferation, and Tumorigenesis in Colon. Cancer Res 2017;77:3352-63. [Crossref] [PubMed]
- Venkateswaran V, Haddad AQ, Fleshner NE, et al. Association of diet-induced hyperinsulinemia with accelerated growth of prostate cancer (LNCaP) xenografts. J Natl Cancer Inst 2007;99:1793-800. [Crossref] [PubMed]
- Rehm S, Ward JM. Quantitative analysis of alveolar type II cell tumors in mice by whole lung serial and step sections. Toxicol Pathol 1989;17:737-42. [Crossref] [PubMed]
- Rehm S, Devor DE, Henneman JR, et al. Origin of spontaneous and transplacentally induced mouse lung tumors from alveolar type II cells. Exp Lung Res 1991;17:181-95. [Crossref] [PubMed]
- Kimura K. Progression of pulmonary tumor in mice. 1. Histological studies of primary and transplanted pulmonary tumors. Acta Pathol Jpn 1971;21:13-56. [PubMed]
- Rehm S, Ward JM, ten Have-Opbroek AA, et al. Mouse papillary lung tumors transplacentally induced by N-nitrosoethylurea: evidence for alveolar type II cell origin by comparative light microscopic, ultrastructural, and immunohistochemical studies. Cancer Res 1988;48:148-60. [PubMed]
- Amaral-Mendes JJ. Histopathology of primary lung tumours in the mouse. J Pathol 1969;97:415-27. [Crossref] [PubMed]
- Xing D, Berrebi AA, Liu C, et al. An Epithelioid Smooth Muscle Neoplasm Mimicking a Signet Ring Cell Carcinoma in the Ovary. Int J Gynecol Pathol 2019;38:464-9. [Crossref] [PubMed]
- DeCoste R, Offman SL. Signet Ring Stromal Cell Tumor: A Legitimate (Benign) Mimic of Krukenberg Tumor. Arch Pathol Lab Med 2018;142:1289-91. [Crossref] [PubMed]
- Farhat NA, Onenerk AM, Krane JF, et al. Primary Benign and Malignant Thyroid Neoplasms With Signet Ring Cells: Cytologic, Histologic, and Molecular Features. Am J Clin Pathol 2017;148:251-8. [Crossref] [PubMed]
- Gnepp DR. Mucinous myoepithelioma, a recently described new myoepithelioma variant. Head Neck Pathol 2013;7 Suppl 1:S85-9. [Crossref] [PubMed]
- Vang R, Bague S, Tavassoli FA, et al. Signet-ring stromal tumor of the ovary: clinicopathologic analysis and comparison with Krukenberg tumor. Int J Gynecol Pathol 2004;23:45-51. [Crossref] [PubMed]
- Rehm S, Ward JM, Anderson LM, et al. Transplacental induction of mouse lung tumors: stage of fetal organogenesis in relation to frequency, morphology, size, and neoplastic progression of N-nitrosoethylurea-induced tumors. Toxicol Pathol 1991;19:35-46. [Crossref] [PubMed]
- Palmer KC. Clara cell adenomas of the mouse lung. Interaction with alveolar type 2 cells. Am J Pathol 1985;120:455-63. [PubMed]
- Branstetter DG, Moseley PP. Effect of lung development on the histological pattern of lung tumors induced by ethylnitrosourea in the C3HeB/FeJ mouse. Exp Lung Res 1991;17:169-79. [Crossref] [PubMed]
- Dai LJ, Huang YL, Zhou MY, et al. Comparative analysis of the impact of intraperitoneal injection of acidic phosphate buffer solution at the late stage of gestation on the reproductive physiology in two inbred mouse strains. Chin J Comp Med 2012;20:27-32.


